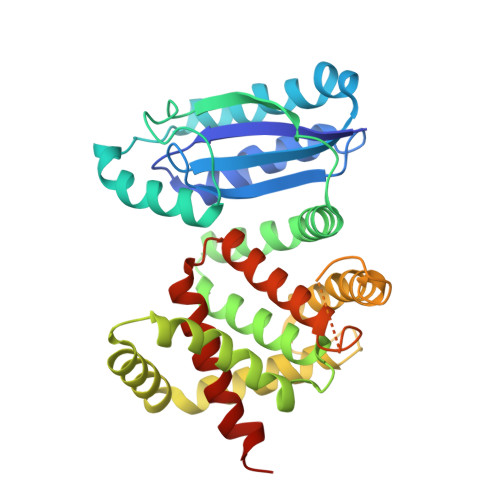
Structural insights into enzymatic [4+2] aza-cycloaddition in thiopeptide antibiotic biosynthesis.
Cogan, D.P., Hudson, G.A., Zhang, Z., Pogorelov, T.V., van der Donk, W.A., Mitchell, D.A., Nair, S.K.(2017) Proc Natl Acad Sci U S A 114: 12928-12933
- PubMed: 29158402
- DOI: https://doi.org/10.1073/pnas.1716035114
- Primary Citation of Related Structures:
5W98, 5W99, 5WA3, 5WA4 - PubMed Abstract:
The [4+2] cycloaddition reaction is an enabling transformation in modern synthetic organic chemistry, but there are only limited examples of dedicated natural enzymes that can catalyze this transformation. Thiopeptides (or more formally thiazolyl peptides) are a class of thiazole-containing, highly modified, macrocyclic secondary metabolites made from ribosomally synthesized precursor peptides. The characteristic feature of these natural products is a six-membered nitrogenous heterocycle that is assembled via a formal [4+2] cycloaddition between two dehydroalanine (Dha) residues. This heteroannulation is entirely contingent on enzyme activity, although the mechanism of the requisite pyridine/dehydropiperidine synthase remains to be elucidated. The unusual aza -cylic product is distinct from the more common carbocyclic products of synthetic and biosynthetic [4+2] cycloaddition reactions. To elucidate the mechanism of cycloaddition, we have determined atomic resolution structures of the pyridine synthases involved in the biosynthesis of the thiopeptides thiomuracin (TbtD) and GE2270A (PbtD), in complex with substrates and product analogs. Structure-guided biochemical, mutational, computational, and binding studies elucidate active-site features that explain how orthologs can generate rigid macrocyclic scaffolds of different sizes. Notably, the pyridine synthases show structural similarity to the elimination domain of lanthipeptide dehydratases, wherein insertions of secondary structural elements result in the formation of a distinct active site that catalyzes different chemistry. Comparative analysis identifies other catalysts that contain a shared core protein fold but whose active sites are located in entirely different regions, illustrating a principle predicted from efforts in de novo protein design.
- Department of Biochemistry, University of Illinois, Urbana, IL 61801.
Organizational Affiliation: