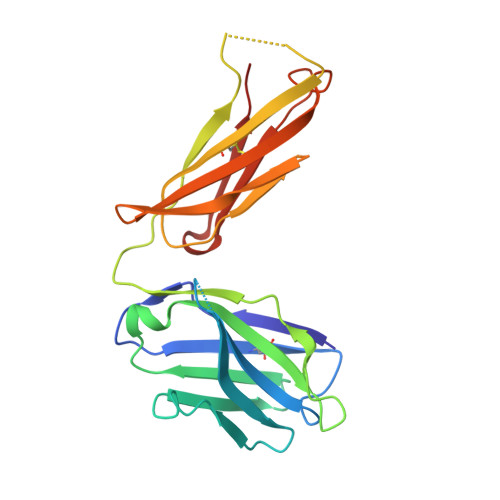
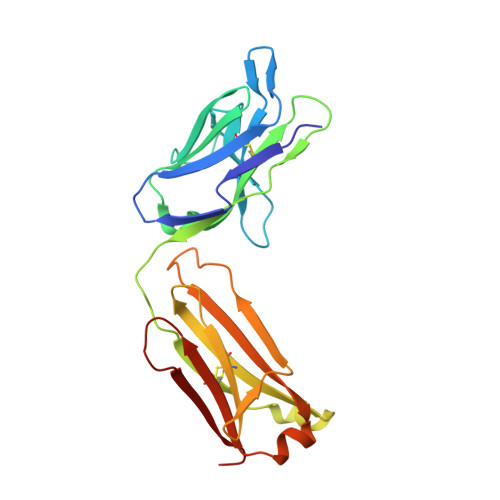
Escape of Hepatitis C Virus from Epitope I Neutralization Increases Sensitivity of Other Neutralization Epitopes.
Gu, J., Hardy, J., Boo, I., Vietheer, P., McCaffrey, K., Alhammad, Y., Chopra, A., Gaudieri, S., Poumbourios, P., Coulibaly, F., Drummer, H.E.(2018) J Virol 92
- PubMed: 29467319
- DOI: https://doi.org/10.1128/JVI.02066-17
- Primary Citation of Related Structures:
5VXR - PubMed Abstract:
The hepatitis C virus (HCV) E2 glycoprotein is a major target of the neutralizing antibody (nAb) response, with multiple type-specific and broadly neutralizing antibody (bnAb) epitopes identified. The 412-to-423 region can generate bnAbs that block interaction with the cell surface receptor CD81, with activity toward multiple HCV genotypes. In this study, we reveal the structure of rodent monoclonal antibody 24 (MAb24) with an extensive contact area toward a peptide spanning the 412-to-423 region. The crystal structure of the MAb24-peptide 412-to-423 complex reveals the paratope bound to a peptide hairpin highly similar to that observed with human MAb HCV1 and rodent MAb AP33, but with a different angle of approach. In viral outgrowth experiments, we demonstrated three distinct genotype 2a viral populations that acquired resistance to MAb24 via N415D, N417S, and N415D/H386R mutations. Importantly, the MAb24-resistant viruses exhibited significant increases in sensitivity to the majority of bnAbs directed to epitopes within the 412-to-423 region and in additional antigenic determinants located within E2 and the E1E2 complex. This study suggests that modification of N415 causes a global change in glycoprotein structure that increases its vulnerability to neutralization by other antibodies. This finding suggests that in the context of an antibody response to viral infection, acquisition of escape mutations in the 412-to-423 region renders the virus more susceptible to neutralization by other specificities of nAbs, effectively reducing the immunological fitness of the virus. A vaccine for HCV that generates polyspecific humoral immunity with specificity for the 412-to-423 region and at least one other region of E2 is desirable. IMPORTANCE Understanding how antibodies neutralize hepatitis C virus (HCV) is essential for vaccine development. This study reveals for the first time that when HCV develops resistance to a major class of bnAbs targeting the 412-to-423 region of E2, this results in a concomitant increase in sensitivity to neutralization by a majority of other bnAb specificities. Vaccines for the prevention of HCV infection should therefore generate bnAbs directed toward the 412-to-423 region of E2 and additional bnAb epitopes within the viral glycoproteins.
- Virus Entry and Vaccines Laboratory, Life Sciences, Burnet Institute, Melbourne, Australia.
Organizational Affiliation: