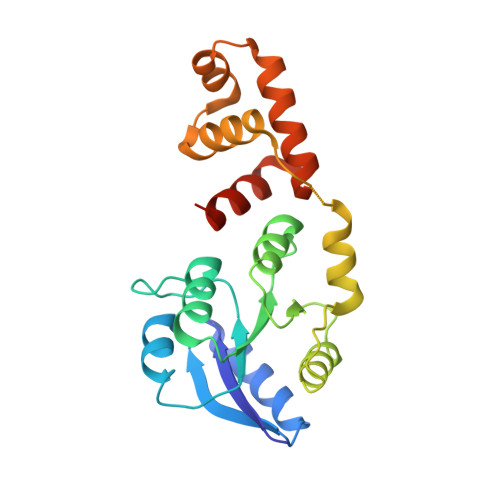
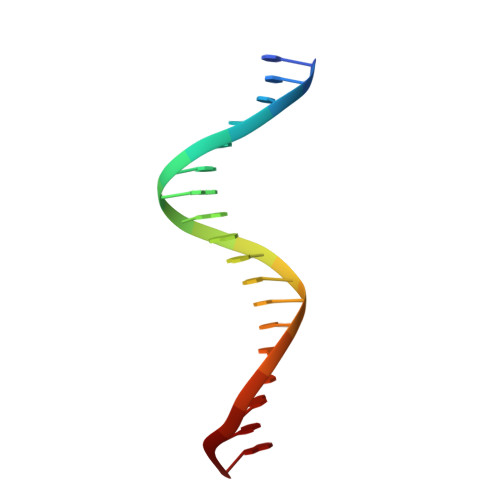
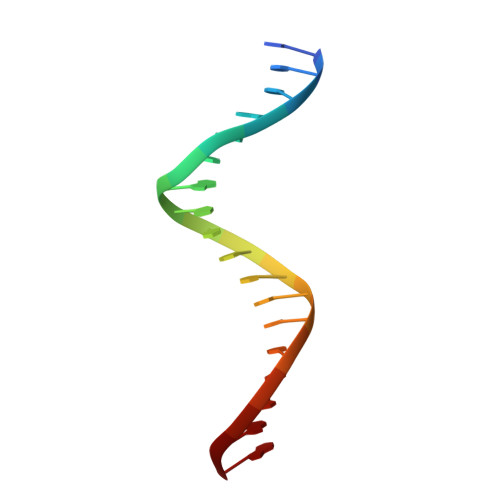
Structural Basis for DNA Recognition by the Two-Component Response Regulator RcsB.
Filippova, E.V., Zemaitaitis, B., Aung, T., Wolfe, A.J., Anderson, W.F.(2018) mBio 9
- PubMed: 29487239
- DOI: https://doi.org/10.1128/mBio.01993-17
- Primary Citation of Related Structures:
5VXN, 5W43 - PubMed Abstract:
RcsB is a highly conserved transcription regulator of the Rcs phosphorelay system, a complex two-component signal transduction system (N. Majdalani and S. Gottesman, Annu Rev Microbiol 59:379-405, 2005; A. J. Wolfe, Curr Opin Microbiol 13:204-209, 2010, https://doi.org/10.1016/j.mib.2010.01.002; D. J. Clarke, Future Microbiol 5:1173-1184, 2010, https://doi.org/10.2217/fmb.10.83). RcsB plays an important role in virulence and pathogenicity in human hosts by regulating biofilm formation. RcsB can regulate transcription alone or together with its auxiliary transcription regulators by forming heterodimers. This complexity allows RcsB to regulate transcription of more than 600 bacterial genes in response to different stresses (D. Wang et al., Mol Plant Microbe Interact 25:6-17, 2012, https://doi.org/10.1094/MPMI-08-11-0207). Despite increasing knowledge of RcsB importance, molecular mechanisms that drive the ability of RcsB to control transcription of a large number of genes remain unclear. Here, we present crystal structures of unphosphorylated RcsB in complex with the consensus DNA-binding sequence of 22-mer (DNA22) and 18-mer (DNA18) of the flhDC operon from Escherichia coli determined at 3.15- and 3.37-Å resolution, respectively. The results of our structural analysis combined with the results of in vitro binding assays provide valuable insights to the protein regulatory mechanism, demonstrate how RcsB recognizes target DNA sequences, and reveal a unique oligomeric state that allows RcsB to form homo- and heterodimers. This information will help us understand the complex mechanisms of transcriptional regulation by RcsB in bacteria. IMPORTANCE RcsB is a well-studied two-component response regulator of the Rcs phosphorelay system, conserved within the family Enterobacteriaceae , which includes many pathogens. It is a global regulator, controlling more than 5% of bacterial genes associated with capsule biosynthesis, flagellar biogenesis, cell wall biosynthesis, antibiotic resistance, biofilm formation, and virulence in pathogens. Knowledge of RcsB structure represents a unique opportunity to explore mechanisms that regulate the Rcs phosphorelay system and its role in the family Enterobacteriaceae .
- Center for Structural Genomics of Infectious Diseases, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA e-filippova@northwestern.edu wf-anderson@northwestern.edu.
Organizational Affiliation: