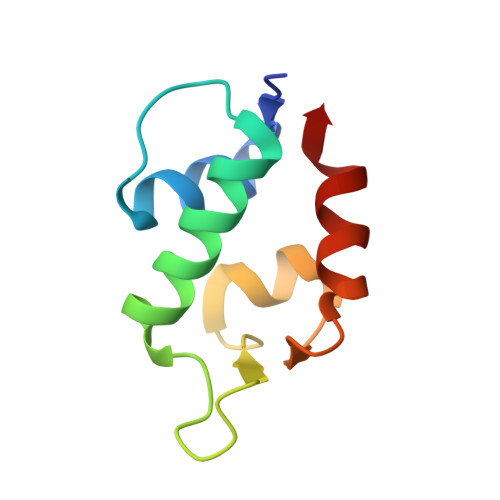
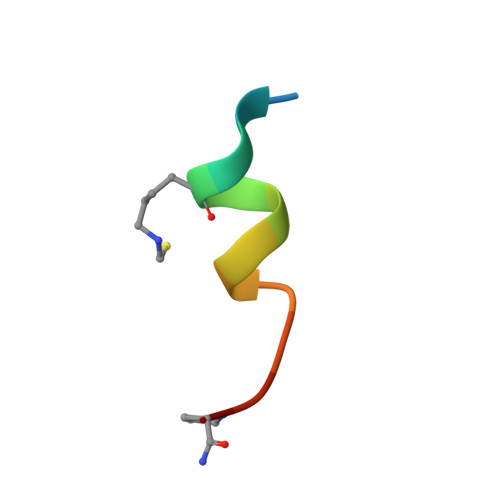
Dithiocarbamate-inspired side chain stapling chemistry for peptide drug design.
Li, X., Tolbert, W.D., Hu, H.G., Gohain, N., Zou, Y., Niu, F., He, W.X., Yuan, W., Su, J.C., Pazgier, M., Lu, W.(2019) Chem Sci 10: 1522-1530
- PubMed: 30809370
- DOI: https://doi.org/10.1039/c8sc03275k
- Primary Citation of Related Structures:
5VK0, 5VK1 - PubMed Abstract:
Two major pharmacological hurdles severely limit the widespread use of small peptides as therapeutics: poor proteolytic stability and membrane permeability. Importantly, low aqueous solubility also impedes the development of peptides for clinical use. Various elaborate side chain stapling chemistries have been developed for α-helical peptides to circumvent this problem, with considerable success in spite of inevitable limitations. Here we report a novel peptide stapling strategy based on the dithiocarbamate chemistry linking the side chains of residues Lys( i ) and Cys( i + 4) of unprotected peptides and apply it to a series of dodecameric peptide antagonists of the p53-inhibitory oncogenic proteins MDM2 and MDMX. Crystallographic studies of peptide-MDM2/MDMX complexes structurally validated the chemoselectivity of the dithiocarbamate staple bridging Lys and Cys at ( i , i + 4) positions. One dithiocarbamate-stapled PMI derivative, DTC PMI, showed a 50-fold stronger binding to MDM2 and MDMX than its linear counterpart. Importantly, in contrast to PMI and its linear derivatives, the DTC PMI peptide actively traversed the cell membrane and killed HCT116 tumor cells in vitro by activating the tumor suppressor protein p53. Compared with other known stapling techniques, our solution-based DTC stapling chemistry is simple, cost-effective, regio-specific and environmentally friendly, promising an important new tool for the development of peptide therapeutics with improved pharmacological properties including aqueous solubility, proteolytic stability and membrane permeability.
- School of Pharmacy , Second Military Medical University , Shanghai 200433 , China.
Organizational Affiliation: