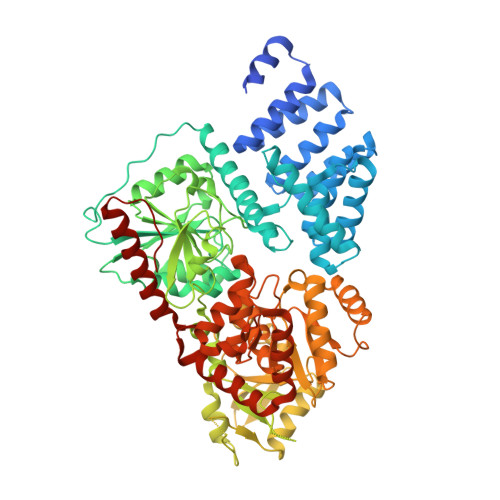
Electrophilic probes for deciphering substrate recognition by O-GlcNAc transferase.
Hu, C.W., Worth, M., Fan, D., Li, B., Li, H., Lu, L., Zhong, X., Lin, Z., Wei, L., Ge, Y., Li, L., Jiang, J.(2017) Nat Chem Biol 13: 1267-1273
- PubMed: 29058723
- DOI: https://doi.org/10.1038/nchembio.2494
- Primary Citation of Related Structures:
5VIE, 5VIF - PubMed Abstract:
O-linked β-N-acetylglucosamine (O-GlcNAc) transferase (OGT) is an essential human glycosyltransferase that adds O-GlcNAc modifications to numerous proteins. However, little is known about the mechanism with which OGT recognizes various protein substrates. Here we report on GlcNAc electrophilic probes (GEPs) to expedite the characterization of OGT-substrate recognition. Data from mass spectrometry, X-ray crystallization, and biochemical and radiolabeled kinetic assays support the application of GEPs to rapidly report the impacts of OGT mutations on protein substrate or sugar binding and to discover OGT residues crucial for protein recognition. Interestingly, we found that the same residues on the inner surface of the N-terminal domain contribute to OGT interactions with different protein substrates. By tuning reaction conditions, a GEP enables crosslinking of OGT with acceptor substrates in situ, affording a unique method to discover genuine substrates that weakly or transiently interact with OGT. Hence, GEPs provide new strategies to dissect OGT-substrate binding and recognition.
- Pharmaceutical Sciences Division, School of Pharmacy, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Organizational Affiliation: