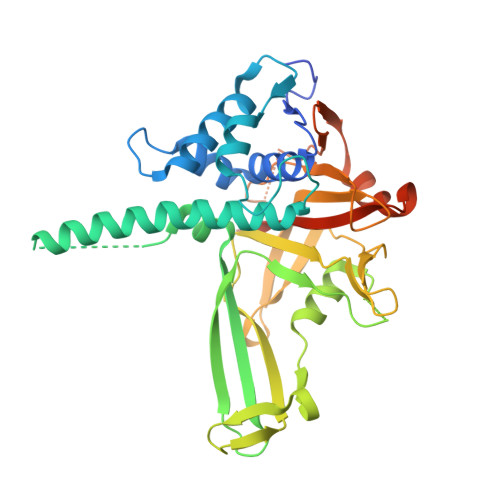
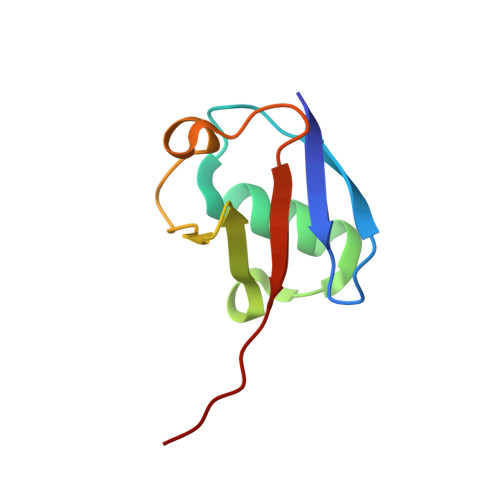
Expansion of DUB functionality generated by alternative isoforms - USP35, a case study.
Leznicki, P., Natarajan, J., Bader, G., Spevak, W., Schlattl, A., Abdul Rehman, S.A., Pathak, D., Weidlich, S., Zoephel, A., Bordone, M.C., Barbosa-Morais, N.L., Boehmelt, G., Kulathu, Y.(2018) J Cell Sci 131
- PubMed: 29685892
- DOI: https://doi.org/10.1242/jcs.212753
- Primary Citation of Related Structures:
5TXK - PubMed Abstract:
Protein ubiquitylation is a dynamic post-translational modification that can be reversed by deubiquitylating enzymes (DUBs). It is unclear how the small number (∼100) of DUBs present in mammalian cells regulate the thousands of different ubiquitylation events. Here, we analysed annotated transcripts of human DUBs and found ∼300 ribosome-associated transcripts annotated as protein coding, which thus increases the total number of DUBs. By using USP35, a poorly studied DUB, as a case study, we provide evidence that alternative isoforms contribute to the functional expansion of DUBs. We show that there are two different USP35 isoforms that localise to different intracellular compartments and have distinct functions. Our results reveal that isoform 1 is an anti-apoptotic factor that inhibits staurosporine- and TNF-related apoptosis-inducing ligand (TRAIL; also known as TNFSF10)-induced apoptosis. In contrast, USP35 isoform 2 is an integral membrane protein of the endoplasmic reticulum (ER) that is also present at lipid droplets. Manipulations of isoform 2 levels cause rapid ER stress, likely through deregulation of lipid homeostasis, and lead to cell death. Our work highlights how alternative isoforms provide functional expansion of DUBs and sets directions for future research.This article has an associated First Person interview with the first author of the paper.
- MRC Protein Phosphorylation and Ubiquitylation Unit, School of Life Sciences, University of Dundee, Dundee DD1 5EH, UK pawel.leznicki@manchester.ac.uk ykulathu@dundee.ac.uk.
Organizational Affiliation: