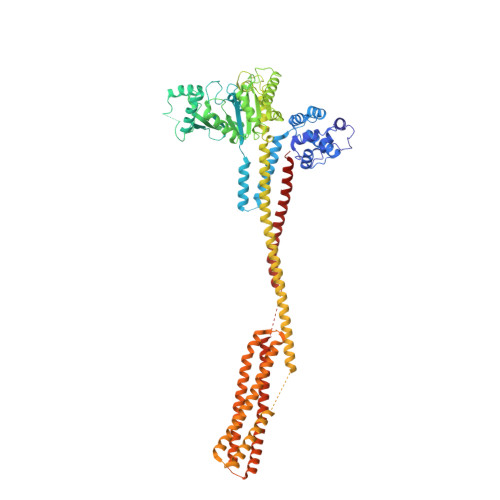
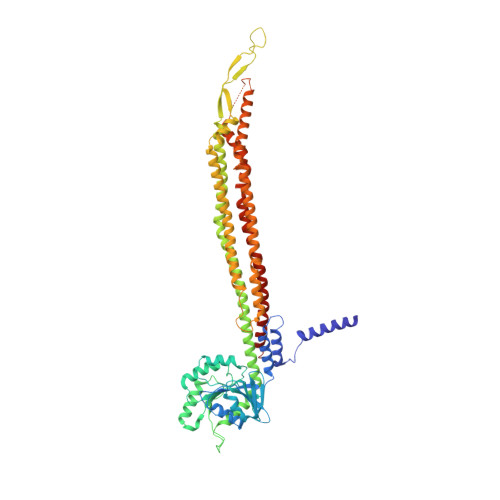
Structural basis for membrane tethering by a bacterial dynamin-like pair.
Liu, J., Noel, J.K., Low, H.H.(2018) Nat Commun 9: 3345-3345
- PubMed: 30131557
- DOI: https://doi.org/10.1038/s41467-018-05523-8
- Primary Citation of Related Structures:
5OWV, 5OXF - PubMed Abstract:
Dynamin-like proteins (DLPs) are large GTPases that restructure membrane. DLPs such as the mitofusins form heterotypic oligomers between isoform pairs that bridge and fuse opposing membranes. In bacteria, heterotypic oligomerisation may also be important for membrane remodelling as most DLP genes are paired within operons. How DLPs tether opposing membranes is unknown. Here we show the crystal structure of a DLP heterotypic pair from the pathogen Campylobacter jejuni. A 2:2 stoichiometric tetramer is observed where heterodimers, conjoined by a random coil linker, assemble back-to-back to form a tripartite DLP chain with extreme flexibility. In vitro, tetramerisation triggers GTPase activity and induces lipid binding. Liposomes are readily tethered and form tubes at high tetramer concentration. Our results provide a direct mechanism for the long-range binding and bridging of opposing membranes by a bacterial DLP pair. They also provide broad mechanistic and structural insights that are relevant to other heterotypic DLP complexes.
- Department of Life Sciences, Imperial College, London, SW7 2AZ, UK.
Organizational Affiliation: