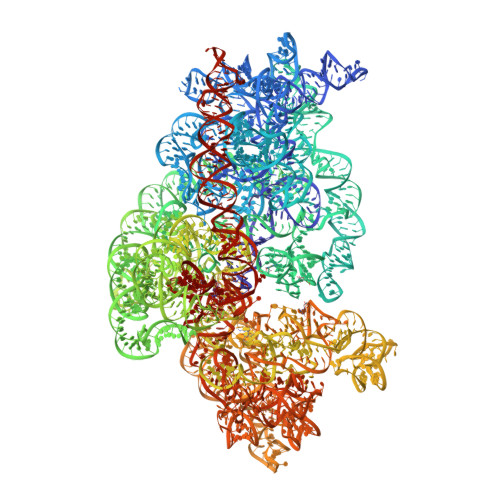
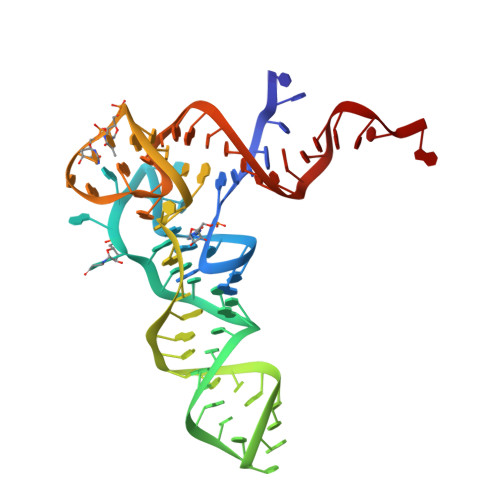
Structure of a 30S pre-initiation complex stalled by GE81112 reveals structural parallels in bacterial and eukaryotic protein synthesis initiation pathways.
Lopez-Alonso, J.P., Fabbretti, A., Kaminishi, T., Iturrioz, I., Brandi, L., Gil-Carton, D., Gualerzi, C.O., Fucini, P., Connell, S.R.(2017) Nucleic Acids Res 45: 2179-2187
- PubMed: 27986852
- DOI: https://doi.org/10.1093/nar/gkw1251
- Primary Citation of Related Structures:
5ME0, 5ME1 - PubMed Abstract:
In bacteria, the start site and the reading frame of the messenger RNA are selected by the small ribosomal subunit (30S) when the start codon, typically an AUG, is decoded in the P-site by the initiator tRNA in a process guided and controlled by three initiation factors. This process can be efficiently inhibited by GE81112, a natural tetrapeptide antibiotic that is highly specific toward bacteria. Here GE81112 was used to stabilize the 30S pre-initiation complex and obtain its structure by cryo-electron microscopy. The results obtained reveal the occurrence of changes in both the ribosome conformation and initiator tRNA position that may play a critical role in controlling translational fidelity. Furthermore, the structure highlights similarities with the early steps of initiation in eukaryotes suggesting that shared structural features guide initiation in all kingdoms of life.
- Structural Biology Unit, CIC bioGUNE, Parque Tecnológico de Bizkaia, 48160 Derio, Bizkaia, Spain.
Organizational Affiliation: