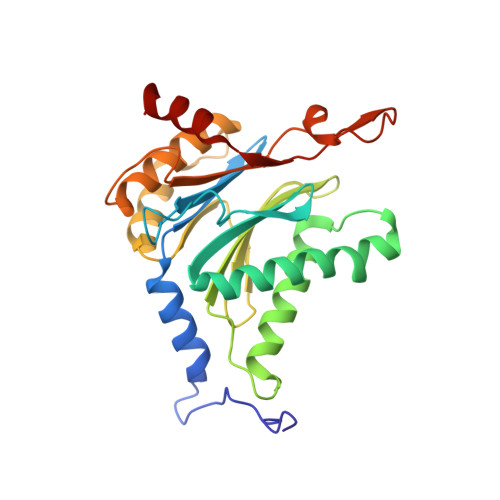
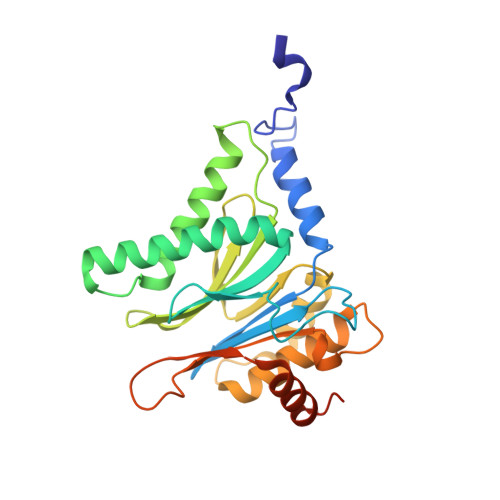
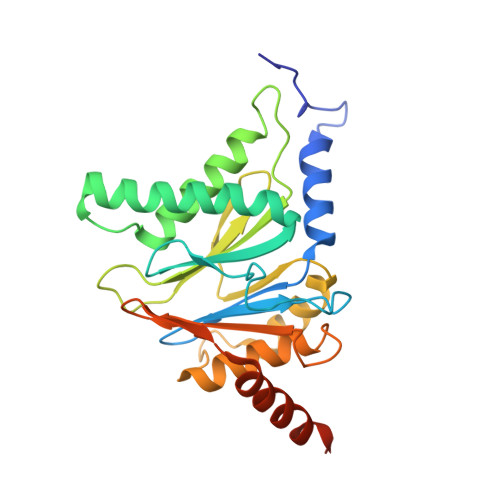
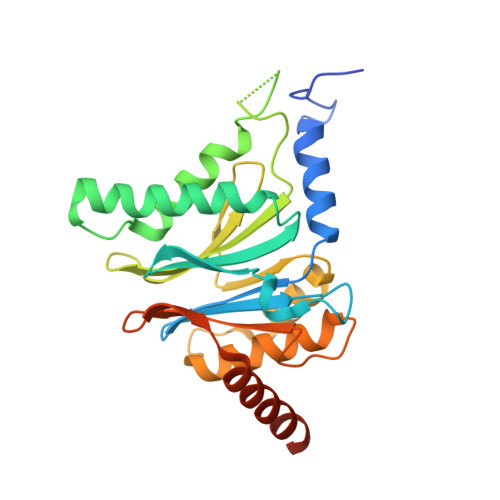
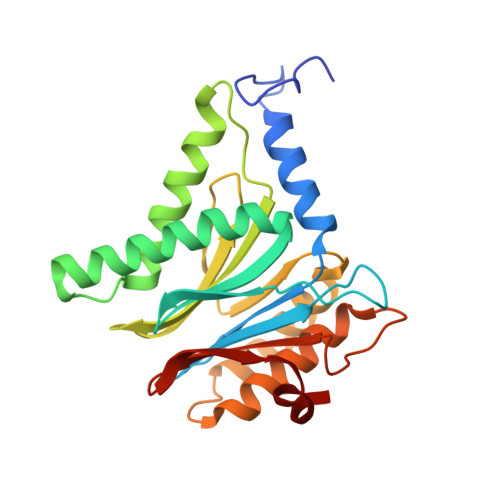
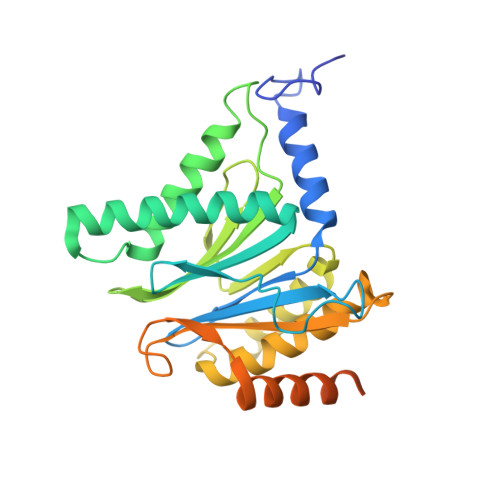
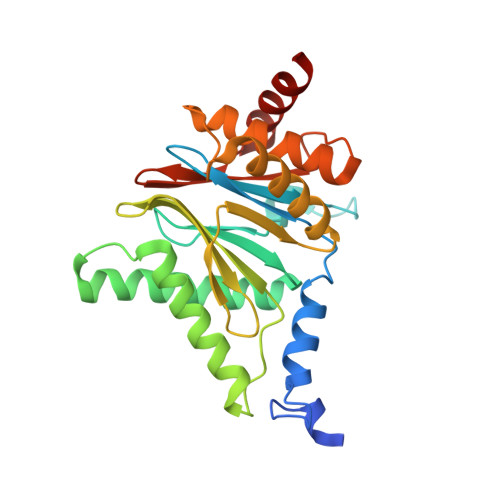
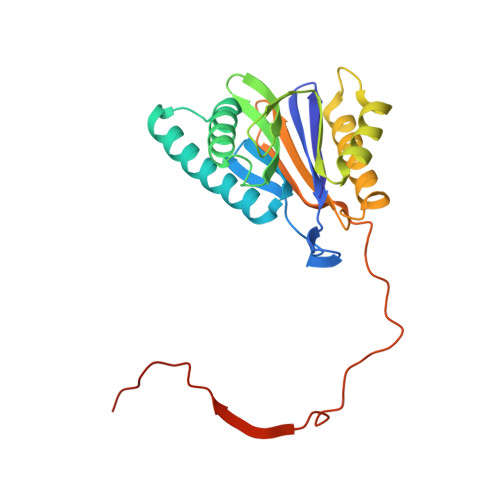
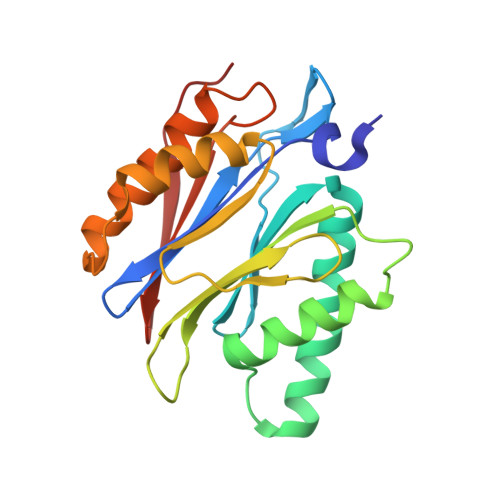
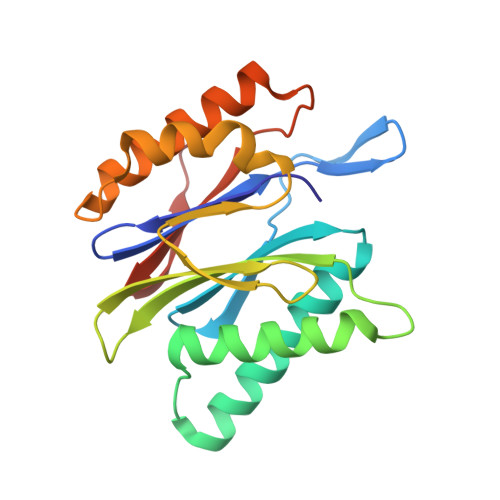
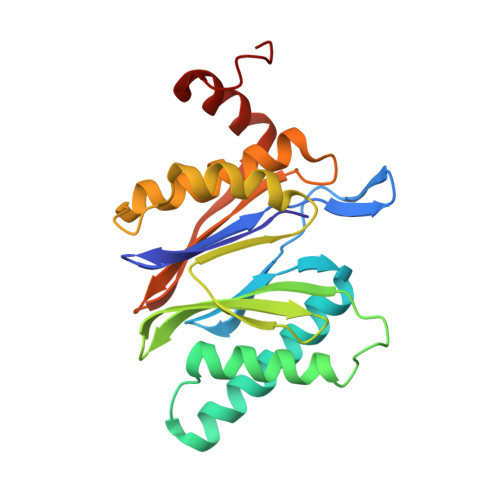
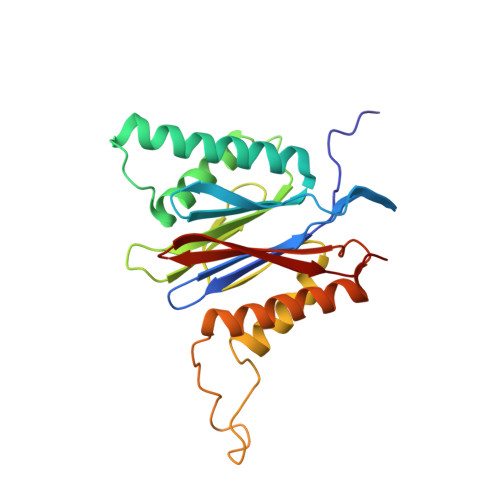
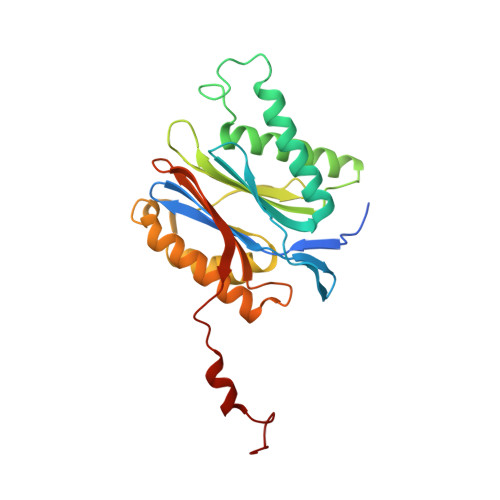
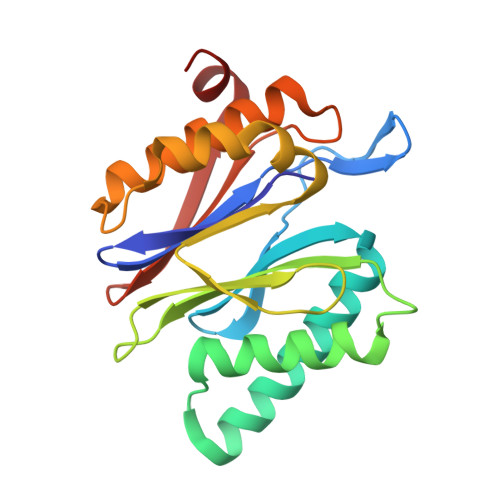
A humanized yeast proteasome identifies unique binding modes of inhibitors for the immunosubunit beta 5i.
Huber, E.M., Heinemeyer, W., de Bruin, G., Overkleeft, H.S., Groll, M.(2016) EMBO J 35: 2602-2613
- PubMed: 27789522
- DOI: https://doi.org/10.15252/embj.201695222
- Primary Citation of Related Structures:
5L52, 5L54, 5L55, 5L5A, 5L5B, 5L5D, 5L5E, 5L5F, 5L5H, 5L5I, 5L5J, 5L5O, 5L5P, 5L5Q, 5L5R, 5L5S, 5L5T, 5L5U, 5L5V, 5L5W, 5L5X, 5L5Y, 5L5Z, 5L60, 5L61, 5L62, 5L63, 5L64, 5L65, 5L66, 5L67, 5L68, 5L69, 5L6A, 5L6B, 5L6C, 5LTT - PubMed Abstract:
Inhibition of the immunoproteasome subunit β5i alleviates autoimmune diseases in preclinical studies and represents a promising new anti-inflammatory therapy. However, the lack of structural data on the human immunoproteasome still hampers drug design. Here, we systematically determined the potency of seven α' β' epoxyketone inhibitors with varying N-caps and P3-stereochemistry for mouse/human β5c/β5i and found pronounced differences in their subunit and species selectivity. Using X-ray crystallography, the compounds were analyzed for their modes of binding to chimeric yeast proteasomes that incorporate key parts of human β5c, human β5i or mouse β5i and the neighboring β6 subunit. The structural data reveal exceptional conformations for the most selective human β5i inhibitors and highlight subtle structural differences as the major reason for the observed species selectivity. Altogether, the presented results validate the humanized yeast proteasome as a powerful tool for structure-based development of β5i inhibitors with potential clinical applications.
- Center for Integrated Protein Science at the Department Chemie, Lehrstuhl für Biochemie Technische Universität München, Garching, Germany.
Organizational Affiliation: