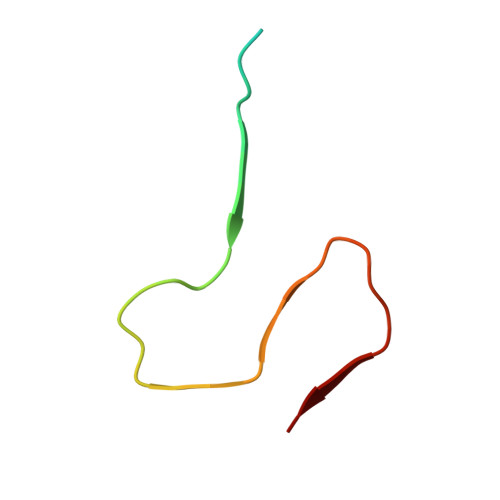
Atomic Resolution Structure of Monomorphic A beta 42 Amyloid Fibrils.
Colvin, M.T., Silvers, R., Ni, Q.Z., Can, T.V., Sergeyev, I., Rosay, M., Donovan, K.J., Michael, B., Wall, J., Linse, S., Griffin, R.G.(2016) J Am Chem Soc 138: 9663-9674
- PubMed: 27355699
- DOI: https://doi.org/10.1021/jacs.6b05129
- Primary Citation of Related Structures:
5KK3 - PubMed Abstract:
Amyloid-β (Aβ) is a 39-42 residue protein produced by the cleavage of the amyloid precursor protein (APP), which subsequently aggregates to form cross-β amyloid fibrils that are a hallmark of Alzheimer's disease (AD). The most prominent forms of Aβ are Aβ1-40 and Aβ1-42, which differ by two amino acids (I and A) at the C-terminus. However, Aβ42 is more neurotoxic and essential to the etiology of AD. Here, we present an atomic resolution structure of a monomorphic form of AβM01-42 amyloid fibrils derived from over 500 (13)C-(13)C, (13)C-(15)N distance and backbone angle structural constraints obtained from high field magic angle spinning NMR spectra. The structure (PDB ID: 5KK3 ) shows that the fibril core consists of a dimer of Aβ42 molecules, each containing four β-strands in a S-shaped amyloid fold, and arranged in a manner that generates two hydrophobic cores that are capped at the end of the chain by a salt bridge. The outer surface of the monomers presents hydrophilic side chains to the solvent. The interface between the monomers of the dimer shows clear contacts between M35 of one molecule and L17 and Q15 of the second. Intermolecular (13)C-(15)N constraints demonstrate that the amyloid fibrils are parallel in register. The RMSD of the backbone structure (Q15-A42) is 0.71 ± 0.12 Å and of all heavy atoms is 1.07 ± 0.08 Å. The structure provides a point of departure for the design of drugs that bind to the fibril surface and therefore interfere with secondary nucleation and for other therapeutic approaches to mitigate Aβ42 aggregation.
- Department of Chemistry and Francis Bitter Magnet Laboratory, Massachusetts Institute of Technology , Cambridge, Massachusetts 02139, United States.
Organizational Affiliation: