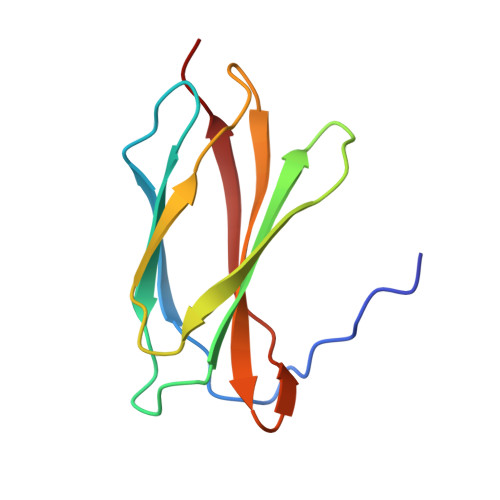
Crystal structure of the second fibronectin type III (FN3) domain from human collagen alpha 1 type XX
Zhao, J., Ren, J., Wang, N., Cheng, Z., Yang, R., Lin, G., Guo, Y., Cai, D., Xie, Y., Zhao, X.(2017) Acta Crystallogr F Struct Biol Commun 73: 695-700
- PubMed: 29199991
- DOI: https://doi.org/10.1107/S2053230X1701648X
- Primary Citation of Related Structures:
5KF4 - PubMed Abstract:
Collagen α1 type XX, which contains fibronectin type III (FN3) repeats involving six FN3 domains (referred to as the FN#1-FN#6 domains), is an unusual member of the fibril-associated collagens with interrupted triple helices (FACIT) subfamily of collagens. The results of standard protein BLAST suggest that the FN3 repeats might contribute to collagen α1 type XX acting as a cytokine receptor. To date, solution NMR structures of the FN#3, FN#4 and FN#6 domains have been determined. To obtain further structural evidence to understand the relationship between the structure and function of the FN3 repeats from collagen α1 type XX, the crystal structure of the FN#2 domain from human collagen α1 type XX (residues Pro386-Pro466; referred to as FN2-HCXX) was solved at 2.5 Å resolution. The crystal structure of FN2-HCXX shows an immunoglobulin-like fold containing a β-sandwich structure, which is formed by a three-stranded β-sheet (β1, β2 and β5) packed onto a four-stranded β-sheet (β3, β4, β6 and β7). Two consensus domains, tencon and fibcon, are structural analogues of FN2-HCXX. Fn8, an FN3 domain from human oncofoetal fibronectin, is the closest structural analogue of FN2-HCXX derived from a naturally occurring sequence. Based solely on the structural similarity of FN2-HCXX to other FN3 domains, the detailed functions of FN2-HCXX and the FN3 repeats in collagen α1 type XX cannot be identified.
- Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Ministry of Education, Chinese Academy of Medical Sciences and Peking Union Medical College, 151 Malianwa North Road, Haidian District, Beijing 100193, People's Republic of China.
Organizational Affiliation: