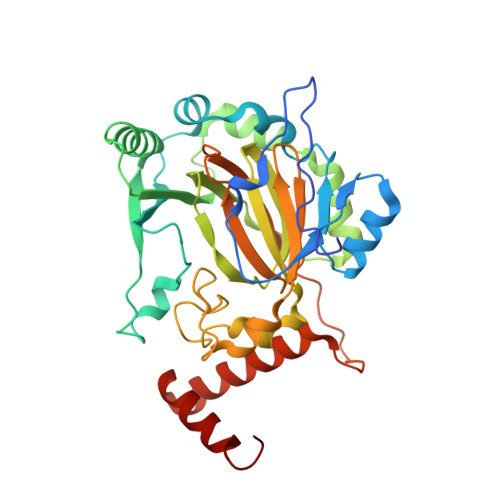
The facial triad in the alpha-ketoglutarate dependent oxygenase FIH: A role for sterics in linking substrate binding to O2 activation.
Hangasky, J.A., Taabazuing, C.Y., Martin, C.B., Eron, S.J., Knapp, M.J.(2016) J Inorg Biochem 166: 26-33
- PubMed: 27815979
- DOI: https://doi.org/10.1016/j.jinorgbio.2016.10.007
- Primary Citation of Related Structures:
5JWP - PubMed Abstract:
The factor inhibiting hypoxia inducible factor-1α (FIH) is a nonheme Fe(II)/αKG oxygenase using a 2-His-1-Asp facial triad. FIH activates O 2 via oxidative decarboxylation of α-ketoglutarate (αKG) to generate an enzyme-based oxidant which hydroxylates the Asn 803 residue within the C-terminal transactivation domain (CTAD) of HIF-1α. Tight coupling of these two sequential reactions requires a structural linkage between the Fe(II) and the substrate binding site to ensure that O 2 activation occurs after substrate binds. We tested the hypothesis that the facial triad carboxylate (Asp 201 ) of FIH linked substrate binding and O 2 binding sites. Asp 201 variants of FIH were constructed and thoroughly characterized in vitro using steady-state kinetics, crystallography, autohydroxylation, and coupling measurements. Our studies revealed each variant activated O 2 with a catalytic efficiency similar to that of wild-type (WT) FIH (k cat aK M(O 2 )=0.17μM -1 min -1 ), but led to defects in the coupling of O 2 activation to substrate hydroxylation. Steady-state kinetics showed similar catalytic efficiencies for hydroxylation by WT-FIH (k cat /K M(CTAD) =0.42μM -1 min -1 ) and D201G (k cat /K M(CTAD) =0.34μM -1 min -1 ); hydroxylation by D201E was greatly impaired, while hydroxylation by D201A was undetectable. Analysis of the crystal structure of the D201E variant revealed steric crowding near the diffusible ligand site supporting a role for sterics from the facial triad carboxylate in the O 2 binding order. Our data support a model in which the facial triad carboxylate Asp 201 provides both steric and polar contacts to favor O 2 access to the Fe(II) only after substrate binds, leading to coupled turnover in FIH and other αKG oxygenases.
- Department of Chemistry, University of Massachusetts, Amherst, United States.
Organizational Affiliation: