Structure of Lys27-linked triubiquitin
Pan, M., Gao, S., Zheng, Y.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ubiquitin | 76 | Homo sapiens | Mutation(s): 0 | 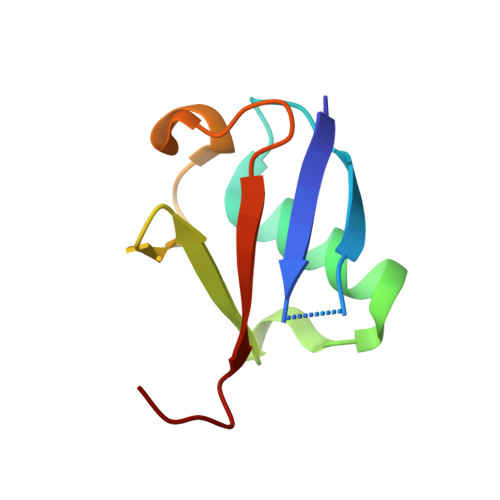 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0CG47 (Homo sapiens) Explore P0CG47 Go to UniProtKB: P0CG47 | |||||
PHAROS: P0CG47 GTEx: ENSG00000170315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0CG47 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| D-ubiquitin | 76 | Homo sapiens | Mutation(s): 0 | 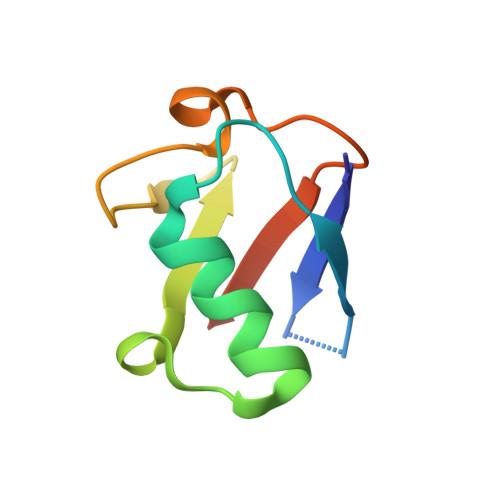 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62987 (Homo sapiens) Explore P62987 Go to UniProtKB: P62987 | |||||
PHAROS: P62987 GTEx: ENSG00000221983 | |||||
Entity Groups | |||||
| UniProt Group | P62987 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 51.758 | α = 90.02 |
| b = 51.749 | β = 118.15 |
| c = 54.977 | γ = 119.95 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |