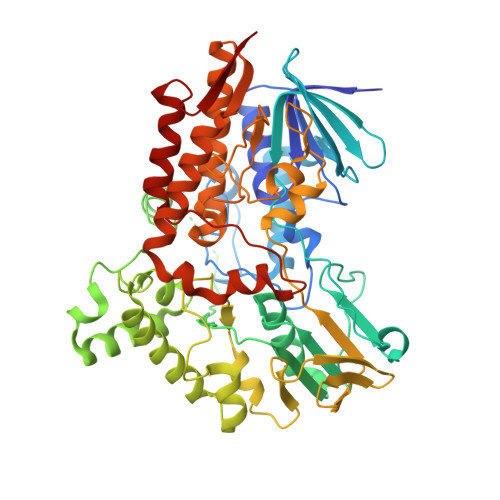
Structural and Catalytic Characterization of a Fungal Baeyer-Villiger Monooxygenase.
Ferroni, F.M., Tolmie, C., Smit, M.S., Opperman, D.J.(2016) PLoS One 11: e0160186-e0160186
- PubMed: 27472055
- DOI: https://doi.org/10.1371/journal.pone.0160186
- Primary Citation of Related Structures:
5J7X - PubMed Abstract:
Baeyer-Villiger monooxygenases (BVMOs) are biocatalysts that convert ketones to esters. Due to their high regio-, stereo- and enantioselectivity and ability to catalyse these reactions under mild conditions, they have gained interest as alternatives to chemical Baeyer-Villiger catalysts. Despite their widespread occurrence within the fungal kingdom, most of the currently characterized BVMOs are from bacterial origin. Here we report the catalytic and structural characterization of BVMOAFL838 from Aspergillus flavus. BVMOAFL838 converts linear and aryl ketones with high regioselectivity. Steady-state kinetics revealed BVMOAFL838 to show significant substrate inhibition with phenylacetone, which was more pronounced at low pH, enzyme and buffer concentrations. Para substitutions on the phenyl group significantly improved substrate affinity and increased turnover frequencies. Steady-state kinetics revealed BVMOAFL838 to preferentially oxidize aliphatic ketones and aryl ketones when the phenyl group are separated by at least two carbons from the carbonyl group. The X-ray crystal structure, the first of a fungal BVMO, was determined at 1.9 Å and revealed the typical overall fold seen in type I bacterial BVMOs. The active site Arg and Asp are conserved, with the Arg found in the "in" position. Similar to phenylacetone monooxygenase (PAMO), a two residue insert relative to cyclohexanone monooxygenase (CHMO) forms a bulge within the active site. Approximately half of the "variable" loop is folded into a short α-helix and covers part of the active site entry channel in the non-NADPH bound structure. This study adds to the current efforts to rationalize the substrate scope of BVMOs through comparative catalytic and structural investigation of different BVMOs.
- Department of Biotechnology, University of the Free State, Bloemfontein, South Africa.
Organizational Affiliation: