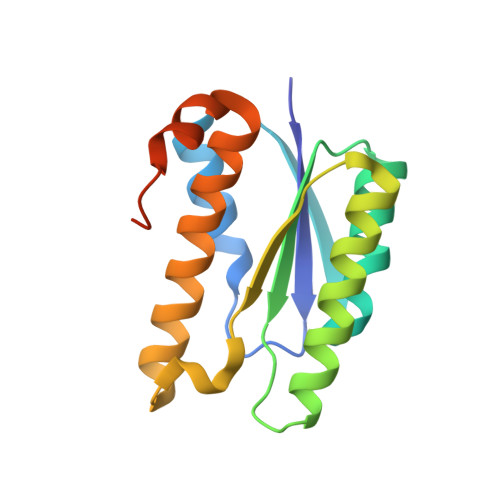
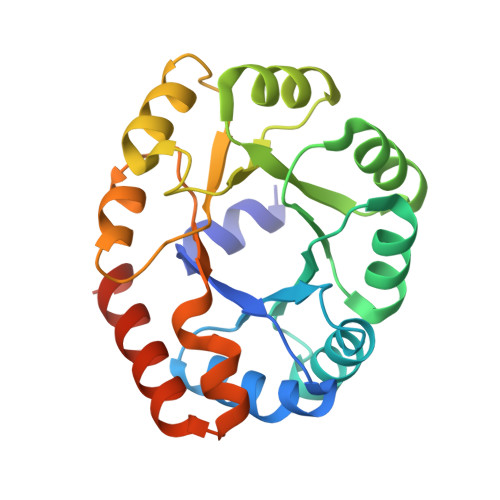
Accurate design of megadalton-scale two-component icosahedral protein complexes.
Bale, J.B., Gonen, S., Liu, Y., Sheffler, W., Ellis, D., Thomas, C., Cascio, D., Yeates, T.O., Gonen, T., King, N.P., Baker, D.(2016) Science 353: 389-394
- PubMed: 27463675
- DOI: https://doi.org/10.1126/science.aaf8818
- Primary Citation of Related Structures:
5IM4, 5IM5, 5IM6 - PubMed Abstract:
Nature provides many examples of self- and co-assembling protein-based molecular machines, including icosahedral protein cages that serve as scaffolds, enzymes, and compartments for essential biochemical reactions and icosahedral virus capsids, which encapsidate and protect viral genomes and mediate entry into host cells. Inspired by these natural materials, we report the computational design and experimental characterization of co-assembling, two-component, 120-subunit icosahedral protein nanostructures with molecular weights (1.8 to 2.8 megadaltons) and dimensions (24 to 40 nanometers in diameter) comparable to those of small viral capsids. Electron microscopy, small-angle x-ray scattering, and x-ray crystallography show that 10 designs spanning three distinct icosahedral architectures form materials closely matching the design models. In vitro assembly of icosahedral complexes from independently purified components occurs rapidly, at rates comparable to those of viral capsids, and enables controlled packaging of molecular cargo through charge complementarity. The ability to design megadalton-scale materials with atomic-level accuracy and controllable assembly opens the door to a new generation of genetically programmable protein-based molecular machines.
- Department of Biochemistry, University of Washington, Seattle, WA 98195, USA. Graduate Program in Molecular and Cellular Biology, University of Washington, Seattle, WA 98195, USA.
Organizational Affiliation: