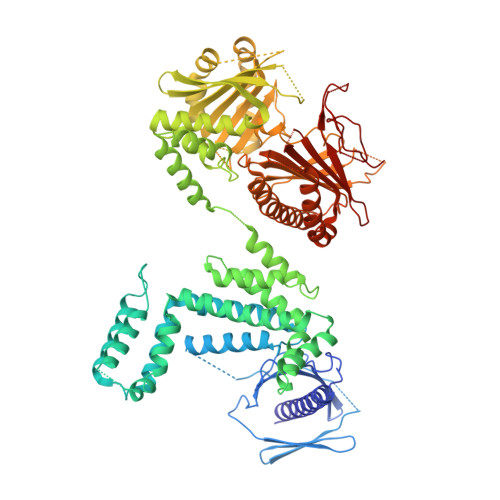
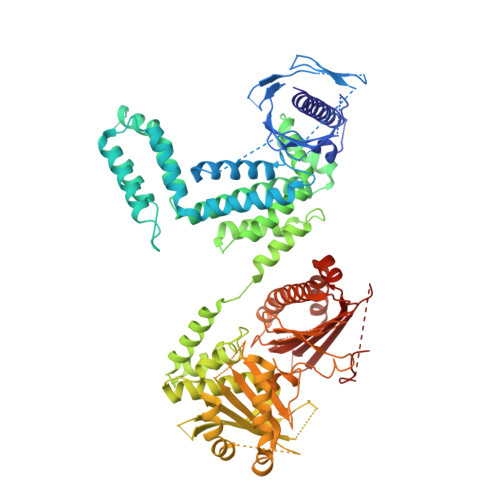
The dynamic organization of fungal acetyl-CoA carboxylase.
Hunkeler, M., Stuttfeld, E., Hagmann, A., Imseng, S., Maier, T.(2016) Nat Commun 7: 11196-11196
- PubMed: 27073141
- DOI: https://doi.org/10.1038/ncomms11196
- Primary Citation of Related Structures:
5I6E, 5I6F, 5I6G, 5I6H, 5I6I, 5I87 - PubMed Abstract:
Acetyl-CoA carboxylases (ACCs) catalyse the committed step in fatty-acid biosynthesis: the ATP-dependent carboxylation of acetyl-CoA to malonyl-CoA. They are important regulatory hubs for metabolic control and relevant drug targets for the treatment of the metabolic syndrome and cancer. Eukaryotic ACCs are single-chain multienzymes characterized by a large, non-catalytic central domain (CD), whose role in ACC regulation remains poorly characterized. Here we report the crystal structure of the yeast ACC CD, revealing a unique four-domain organization. A regulatory loop, which is phosphorylated at the key functional phosphorylation site of fungal ACC, wedges into a crevice between two domains of CD. Combining the yeast CD structure with intermediate and low-resolution data of larger fragments up to intact ACCs provides a comprehensive characterization of the dynamic fungal ACC architecture. In contrast to related carboxylases, large-scale conformational changes are required for substrate turnover, and are mediated by the CD under phosphorylation control.
- Department Biozentrum, University of Basel, Klingelbergstrasse 50/70, 4056 Basel, Switzerland.
Organizational Affiliation: