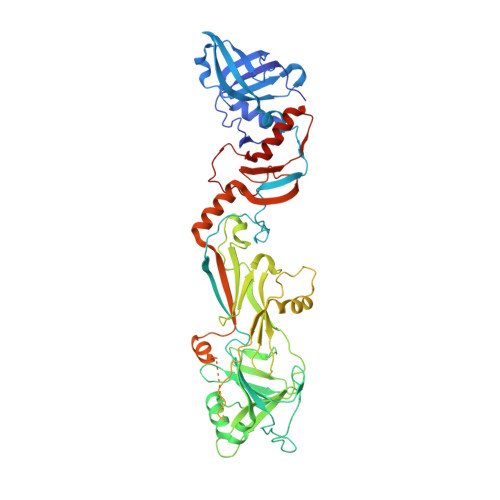
The bacteriophage 29 tail possesses a pore-forming loop for cell membrane penetration.
Xu, J., Gui, M., Wang, D., Xiang, Y.(2016) Nature 534: 544-547
- PubMed: 27309813
- DOI: https://doi.org/10.1038/nature18017
- Primary Citation of Related Structures:
5FB4, 5FB5, 5FEI - PubMed Abstract:
Most bacteriophages are tailed bacteriophages with an isometric or a prolate head attached to a long contractile, long non-contractile, or short non-contractile tail. The tail is a complex machine that plays a central role in host cell recognition and attachment, cell wall and membrane penetration, and viral genome ejection. The mechanisms involved in the penetration of the inner host cell membrane by bacteriophage tails are not well understood. Here we describe structural and functional studies of the bacteriophage ϕ29 tail knob protein gene product 9 (gp9). The 2.0 Å crystal structure of gp9 shows that six gp9 molecules form a hexameric tube structure with six flexible hydrophobic loops blocking one end of the tube before DNA ejection. Sequence and structural analyses suggest that the loops in the tube could be membrane active. Further biochemical assays and electron microscopy structural analyses show that the six hydrophobic loops in the tube exit upon DNA ejection and form a channel that spans the lipid bilayer of the membrane and allows the release of the bacteriophage genomic DNA, suggesting that cell membrane penetration involves a pore-forming mechanism similar to that of certain non-enveloped eukaryotic viruses. A search of other phage tail proteins identified similar hydrophobic loops, which indicates that a common mechanism might be used for membrane penetration by prokaryotic viruses. These findings suggest that although prokaryotic and eukaryotic viruses use apparently very different mechanisms for infection, they have evolved similar mechanisms for breaching the cell membrane.
- Centre for Infectious Diseases Research, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Beijing Advanced Innovation Center for Structural Biology, Department of Basic Medical Sciences, School of Medicine, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: