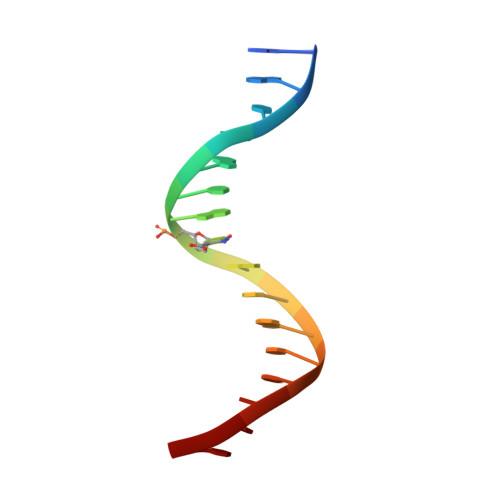
MAX is an epigenetic sensor of 5-carboxylcytosine and is altered in multiple myeloma.
Wang, D., Hashimoto, H., Zhang, X., Barwick, B.G., Lonial, S., Boise, L.H., Vertino, P.M., Cheng, X.(2017) Nucleic Acids Res 45: 2396-2407
- PubMed: 27903915
- DOI: https://doi.org/10.1093/nar/gkw1184
- Primary Citation of Related Structures:
5EYO - PubMed Abstract:
The oncogenic transcription factor MYC and its binding partner MAX regulate gene expression by binding to DNA at enhancer-box (E-box) elements 5΄-CACGTG-3΄. In mammalian genomes, the central E-box CpG has the potential to be methylated at the 5-position of cytosine (5mC), or to undergo further oxidation to the 5-hydroxymethyl (5hmC), 5-formyl (5fC), or 5-carboxyl (5caC) forms. We find that MAX exhibits the greatest affinity for a 5caC or unmodified C-containing E-box, and much reduced affinities for the corresponding 5mC, 5hmC or 5fC forms. Crystallization of MAX with a 5caC modified E-box oligonucleotide revealed that MAX Arg36 recognizes 5caC using a 5caC-Arg-Guanine triad, with the next nearest residue to the carboxylate group being Arg60. In an analysis of >800 primary multiple myelomas, MAX alterations occurred at a frequency of ∼3%, more than half of which were single nucleotide substitutions affecting a basic clamp-like interface important for DNA interaction. Among these, arginines 35, 36 and 60 were the most frequently altered. In vitro binding studies showed that whereas mutation of Arg36 (R36W) or Arg35 (R35H/L) completely abolished DNA binding, mutation of Arg60 (R60Q) significantly reduced DNA binding, but retained a preference for the 5caC modified E-box. Interestingly, MAX alterations define a subset of myeloma patients with lower MYC expression and a better overall prognosis. Together these data indicate that MAX can act as a direct epigenetic sensor of E-box cytosine modification states and that local CpG modification and MAX variants converge to modulate the MAX-MYC transcriptional network.
- Department of Biochemistry, Emory University School of Medicine, Atlanta, GA 30322, USA.
Organizational Affiliation: