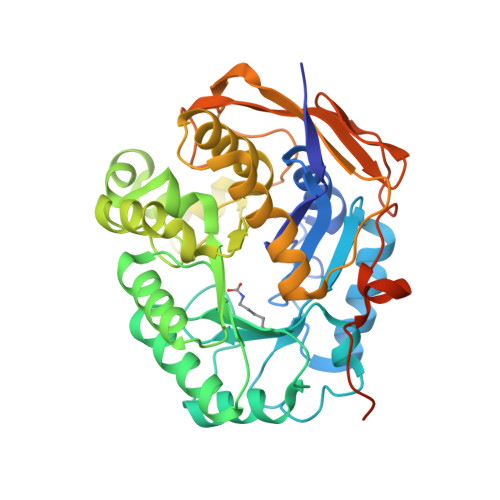
Structure, Functional Characterization and Evolution of the Dihydroorotase Domain of Human Cad.
Grande-Garcia, A., Lallous, N., Diaz-Tejada, C., Ramon-Maiques, S.(2014) Structure 22: 185
- PubMed: 24332717
- DOI: https://doi.org/10.1016/j.str.2013.10.016
- Primary Citation of Related Structures:
4BY3, 4C6B, 4C6C, 4C6D, 4C6E, 4C6F, 4C6I, 4C6J, 4C6K, 4C6L, 4C6M, 4C6N, 4C6O, 4C6P, 4C6Q - PubMed Abstract:
Upregulation of CAD, the multifunctional protein that initiates and controls the de novo biosynthesis of pyrimidines in animals, is essential for cell proliferation. Deciphering the architecture and functioning of CAD is of interest for its potential usage as an antitumoral target. However, there is no detailed structural information about CAD other than that it self-assembles into hexamers of ∼1.5 MDa. Here we report the crystal structure and functional characterization of the dihydroorotase domain of human CAD. Contradicting all assumptions, the structure reveals an active site enclosed by a flexible loop with two Zn²⁺ ions bridged by a carboxylated lysine and a third Zn coordinating a rare histidinate ion. Site-directed mutagenesis and functional assays prove the involvement of the Zn and flexible loop in catalysis. Comparison with homologous bacterial enzymes supports a reclassification of the DHOase family and provides strong evidence against current models of the architecture of CAD.
- Structural Bases of Genome Integrity Group, Structural Biology and Biocomputing Programme, Spanish National Cancer Research Centre (CNIO), 28029 Madrid, Spain.
Organizational Affiliation: