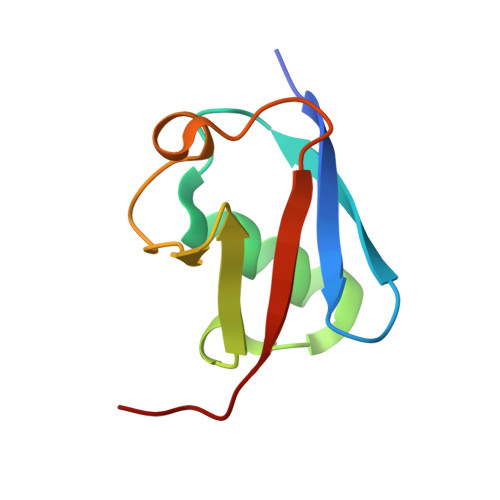
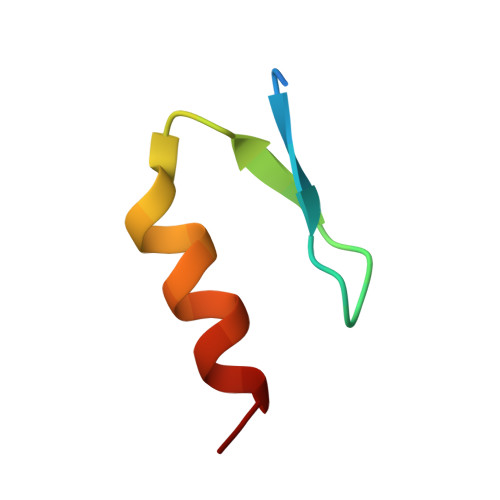
Molecular basis of ubiquitin recognition by the autophagy receptor CALCOCO2
Xie, X., Li, F., Wang, Y., Wang, Y., Lin, Z., Cheng, X., Liu, J., Chen, C., Pan, L.(2015) Autophagy 11: 1775-1789
- PubMed: 26506893
- DOI: https://doi.org/10.1080/15548627.2015.1082025
- Primary Citation of Related Structures:
2MXP, 4XKL - PubMed Abstract:
The autophagy receptor CALCOCO2/NDP52 functions as a bridging adaptor and plays an essential role in the selective autophagic degradation of invading pathogens by specifically recognizing ubiquitin-coated intracellular pathogens and subsequently targeting them to the autophagic machinery; thereby it is required for innate immune defense against a range of infectious pathogens in mammals. However, the mechanistic basis underlying CALCOCO2-mediated specific recognition of ubiqutinated pathogens is still unknown. Here, using biochemical and structural analyses, we demonstrated that the cargo-binding region of CALCOCO2 contains a dynamic unconventional zinc finger as well as a C2H2-type zinc-finger, and only the C2H2-type zinc finger specifically recognizes mono-ubiquitin or poly-ubiquitin chains. In addition to elucidating the specific ubiquitin recognition mechanism of CALCOCO2, the structure of the CALCOCO2 C2H2-type zinc finger in complex with mono-ubiquitin also uncovers a unique zinc finger-binding mode for ubiquitin. Our findings provide mechanistic insight into how CALCOCO2 targets ubiquitin-decorated pathogens for autophagic degradations.
- a State Key Laboratory of Bioorganic and Natural Products Chemistry.
Organizational Affiliation: