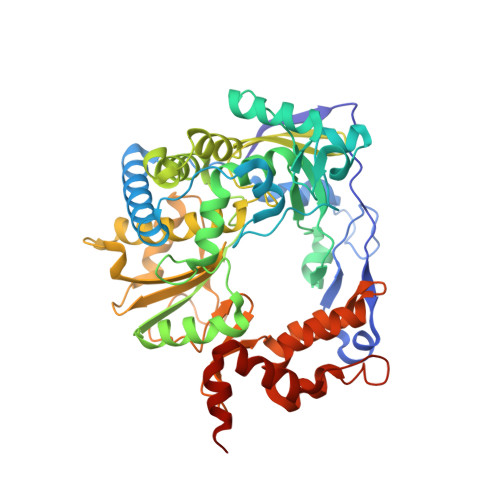
Multifunctionality of a picornavirus polymerase domain: nuclear localization signal and nucleotide recognition.
Ferrer-Orta, C., de la Higuera, I., Caridi, F., Sanchez-Aparicio, M.T., Moreno, E., Perales, C., Singh, K., Sarafianos, S.G., Sobrino, F., Domingo, E., Verdaguer, N.(2015) J Virol 89: 6848-6859
- PubMed: 25903341
- DOI: https://doi.org/10.1128/JVI.03283-14
- Primary Citation of Related Structures:
4WYL, 4WYW, 4WZM, 4WZQ, 4X2B - PubMed Abstract:
The N-terminal region of the foot-and-mouth disease virus (FMDV) 3D polymerase contains the sequence MRKTKLAPT (residues 16 to 24) that acts as a nuclear localization signal. A previous study showed that substitutions K18E and K20E diminished the transport to the nucleus of 3D and 3CD and severely impaired virus infectivity. These residues have also been implicated in template binding, as seen in the crystal structures of different 3D-RNA elongation complexes. Here, we report the biochemical and structural characterization of different mutant polymerases harboring substitutions at residues 18 and 20, in particular, K18E, K18A, K20E, K20A, and the double mutant K18A K20A (KAKA). All mutant enzymes exhibit low RNA binding activity, low processivity, and alterations in nucleotide recognition, including increased incorporation of ribavirin monophosphate (RMP) relative to the incorporation of cognate nucleotides compared with the wild-type enzyme. The structural analysis shows an unprecedented flexibility of the 3D mutant polymerases, including both global rearrangements of the closed-hand architecture and local conformational changes at loop β9-α11 (within the polymerase motif B) and at the template-binding channel. Specifically, in 3D bound to RNA, both K18E and K20E induced the opening of new pockets in the template channel where the downstream templating nucleotide at position +2 binds. The comparisons of free and RNA-bound enzymes suggest that the structural rearrangements may occur in a concerted mode to regulate RNA replication, processivity, and fidelity. Thus, the N-terminal region of FMDV 3D that acts as a nuclear localization signal (NLS) and in template binding is also involved in nucleotide recognition and can affect the incorporation of nucleotide analogues. The study documents multifunctionality of a nuclear localization signal (NLS) located at the N-terminal region of the foot-and-mouth disease viral polymerase (3D). Amino acid substitutions at this polymerase region can impair the transport of 3D to the nucleus, reduce 3D binding to RNA, and alter the relative incorporation of standard nucleoside monophosphate versus ribavirin monophosphate. Structural data reveal that the conformational changes in this region, forming part of the template channel entry, would be involved in nucleotide discrimination. The results have implications for the understanding of viral polymerase function and for lethal mutagenesis mechanisms.
- Institut de Biologia Molecular de Barcelona, CSIC, Parc Científic de Barcelona, Barcelona, Spain.
Organizational Affiliation: