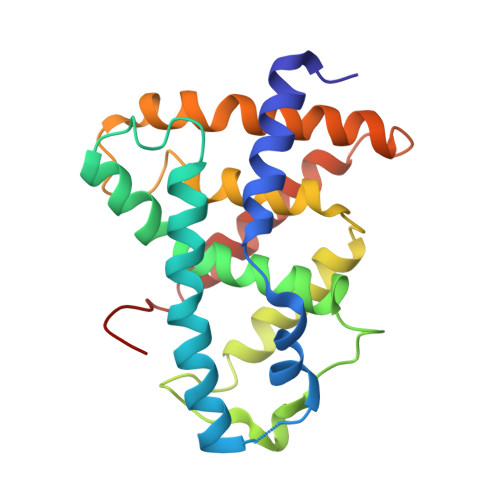
The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism.
Jin, L., Feng, X., Rong, H., Pan, Z., Inaba, Y., Qiu, L., Zheng, W., Lin, S., Wang, R., Wang, Z., Wang, S., Liu, H., Li, S., Xie, W., Li, Y.(2013) Nat Commun 4: 1937
- PubMed: 23728580
- DOI: https://doi.org/10.1038/ncomms2924
- Primary Citation of Related Structures:
4WVD - PubMed Abstract:
Farnesoid X receptor (FXR) has important roles in maintaining bile acid and cholesterol homeostasis. Here we report that the antiparasitic drug ivermectin is a ligand for nuclear FXR. We identify ivermectin using a high-throughput compound library screening and show that it induces the transcriptional activity of the FXR with distinctive properties in modulating coregulator recruitment. The crystal structure of ivermectin complexed with the ligand-binding domain of FXR reveals a unique binding mode of ivermectin in the FXR ligand-binding pocket, including the highly dynamic AF-2 helix and an expanded ligand-binding pocket. Treatment of wild-type mice, but not of FXR-null mice, with ivermectin decreases serum glucose and cholesterol levels, suggesting that ivermectin regulates metabolism through FXR. Our results establish FXR as the first mammalian protein targeted by ivermectin with high selectivity. Considering that ivermectin is a widely used clinical drug, our findings reveal a safe template for the design of novel FXR ligands.
- State Key Laboratory of Cellular Stress Biology, School of Life Sciences, Xiamen University, Fujian 361005, China.
Organizational Affiliation: