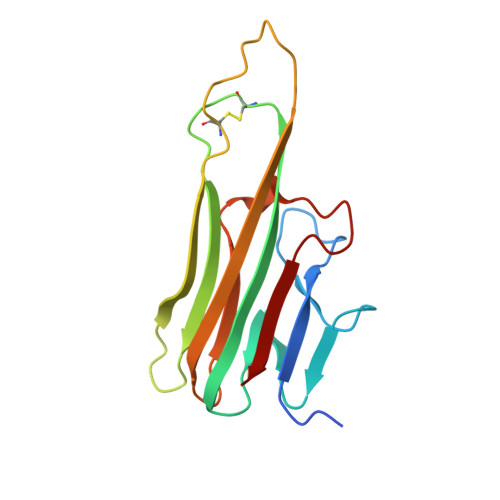
Subunit disassembly and inhibition of TNF alpha by a semi-synthetic bicyclic peptide.
Luzi, S., Kondo, Y., Bernard, E., Stadler, L.K., Vaysburd, M., Winter, G., Holliger, P.(2015) Protein Eng Des Sel 28: 45-52
- PubMed: 25614525
- DOI: https://doi.org/10.1093/protein/gzu055
- Primary Citation of Related Structures:
4TWT - PubMed Abstract:
Macrocyclic peptides are potentially a source of powerful drugs, but their de novo discovery remains challenging. Here we describe the discovery of a high-affinity (Kd = 10 nM) peptide macrocycle (M21) against human tumor necrosis factor-alpha (hTNFα), a key drug target in the treatment of inflammatory disorders, directly from diverse semi-synthetic phage peptide repertoires. The bicyclic peptide M21 (ACPPCLWQVLC) comprises two loops covalently anchored to a 2,4,6-trimethyl-mesitylene core and upon binding induces disassembly of the trimeric TNFα cytokine into dimers and monomers. A 2.9 Å crystal structure of the M21/hTNFα complex reveals the peptide bound to a hTNFα dimer at a normally buried epitope in the trimer interface overlapping the binding site of a previously discovered small molecule ligand (SPD304), which also induces TNF trimer dissociation and synergizes with M21 in the inhibition of TNFα cytotoxicity. The discovery of M21 underlines the potential of semi-synthetic bicyclic peptides as ligands for the discovery of cryptic epitopes, some of which are poorly accessible to antibodies.
- MRC Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge CB2 0QH, UK.
Organizational Affiliation: