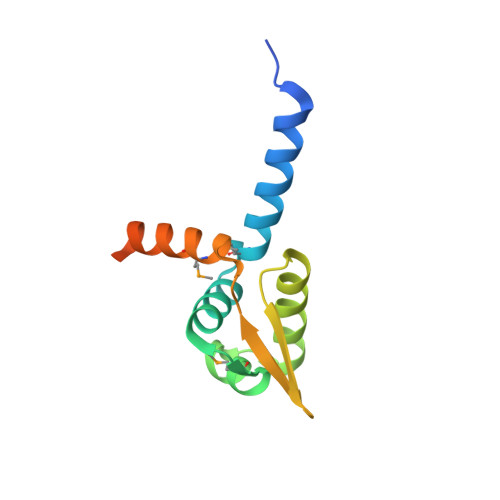
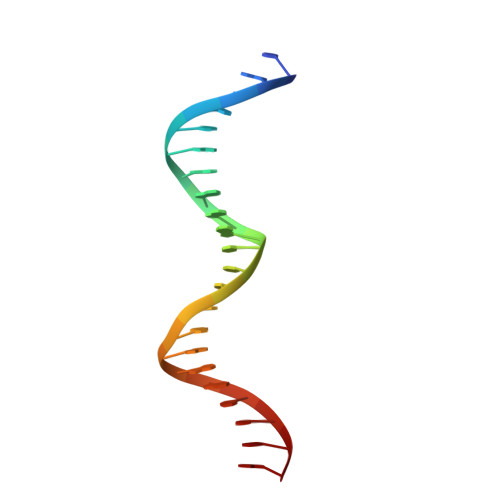
Structural basis for regulation of rhizobial nodulation and symbiosis gene expression by the regulatory protein NolR.
Lee, S.G., Krishnan, H.B., Jez, J.M.(2014) Proc Natl Acad Sci U S A 111: 6509-6514
- PubMed: 24733893
- DOI: https://doi.org/10.1073/pnas.1402243111
- Primary Citation of Related Structures:
4OMY, 4OMZ, 4ON0 - PubMed Abstract:
The symbiosis between rhizobial microbes and host plants involves the coordinated expression of multiple genes, which leads to nodule formation and nitrogen fixation. As part of the transcriptional machinery for nodulation and symbiosis across a range of Rhizobium, NolR serves as a global regulatory protein. Here, we present the X-ray crystal structures of NolR in the unliganded form and complexed with two different 22-base pair (bp) double-stranded operator sequences (oligos AT and AA). Structural and biochemical analysis of NolR reveals protein-DNA interactions with an asymmetric operator site and defines a mechanism for conformational switching of a key residue (Gln56) to accommodate variation in target DNA sequences from diverse rhizobial genes for nodulation and symbiosis. This conformational switching alters the energetic contributions to DNA binding without changes in affinity for the target sequence. Two possible models for the role of NolR in the regulation of different nodulation and symbiosis genes are proposed. To our knowledge, these studies provide the first structural insight on the regulation of genes involved in the agriculturally and ecologically important symbiosis of microbes and plants that leads to nodule formation and nitrogen fixation.
- Department of Biology, Washington University, St. Louis, MO 63130.
Organizational Affiliation: