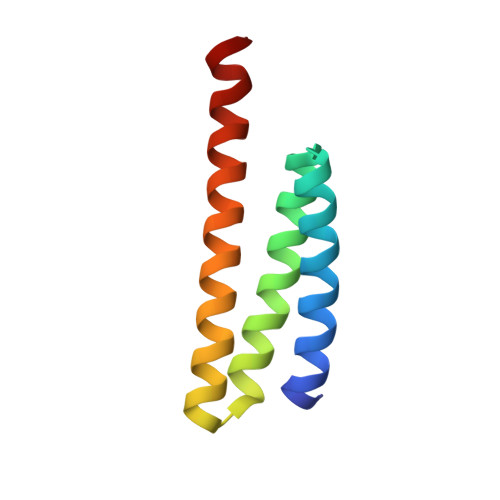
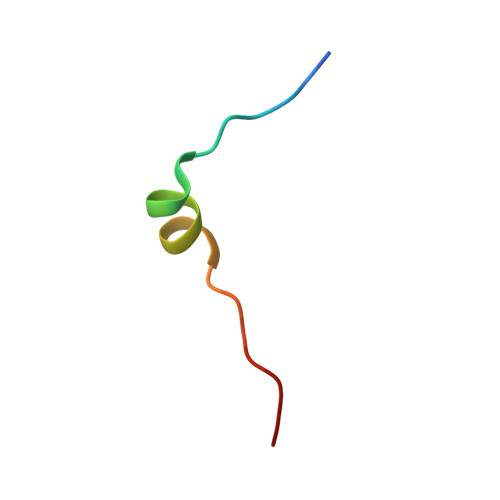
Vfa1 Binds to the N-terminal Microtubule-interacting and Trafficking (MIT) Domain of Vps4 and Stimulates Its ATPase Activity.
Vild, C.J., Xu, Z.(2014) J Biological Chem 289: 10378-10386
- PubMed: 24567329
- DOI: https://doi.org/10.1074/jbc.M113.532960
- Primary Citation of Related Structures:
4NIQ - PubMed Abstract:
The endosomal sorting complexes required for transport (ESCRT) are responsible for multivesicular body biogenesis, membrane abscission during cytokinesis, and retroviral budding. They function as transiently assembled molecular complexes on the membrane, and their disassembly requires the action of the AAA-ATPase Vps4. Vps4 is regulated by a multitude of ESCRT and ESCRT-related proteins. Binding of these proteins to Vps4 is often mediated via the microtubule-interacting and trafficking (MIT) domain of Vps4. Recently, a new Vps4-binding protein Vfa1 was identified in a yeast genetic screen, where overexpression of Vfa1 caused defects in vacuolar morphology. However, the function of Vfa1 and its role in vacuolar biology were largely unknown. Here, we provide the first detailed biochemical and biophysical study of Vps4-Vfa1 interaction. The MIT domain of Vps4 binds to the C-terminal 17 residues of Vfa1. This interaction is of high affinity and greatly stimulates the ATPase activity of Vps4. The crystal structure of the Vps4-Vfa1 complex shows that Vfa1 adopts a canonical MIT-interacting motif 2 structure that has been observed previously in other Vps4-ESCRT interactions. These findings suggest that Vfa1 is a novel positive regulator of Vps4 function.
- Life Sciences Institute and Department of Biological Chemistry, Medical School, University of Michigan, Ann Arbor, Michigan 48109.
Organizational Affiliation: