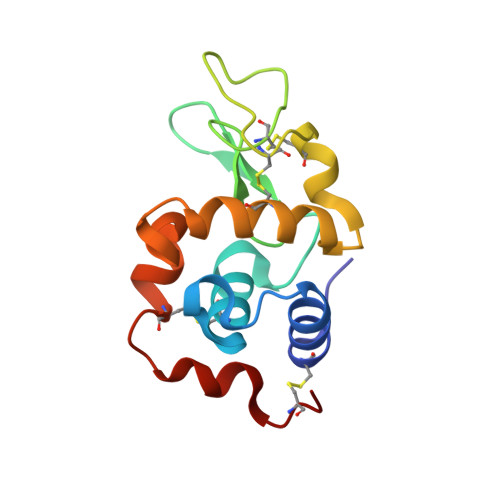
The mode of action of anticancer gold-based drugs: a structural perspective.
Messori, L., Scaletti, F., Massai, L., Cinellu, M.A., Gabbiani, C., Vergara, A., Merlino, A.(2013) Chem Commun (Camb) 49: 10100-10102
- PubMed: 24045294
- DOI: https://doi.org/10.1039/c3cc46400h
- Primary Citation of Related Structures:
4LFP, 4LFX, 4LGK - PubMed Abstract:
The interactions between a few representative gold-based drugs and hen egg white lysozyme were studied by X-ray crystallography. High resolution crystal structures solved for three metallodrug-protein adducts provide valuable insight into the molecular mechanism of these promising metal compounds and the inherent protein metalation processes.
- Department of Chemistry, University of Florence, Via della Lastruccia 3, 50019 Sesto Fiorentino (FI), Italy.
Organizational Affiliation: