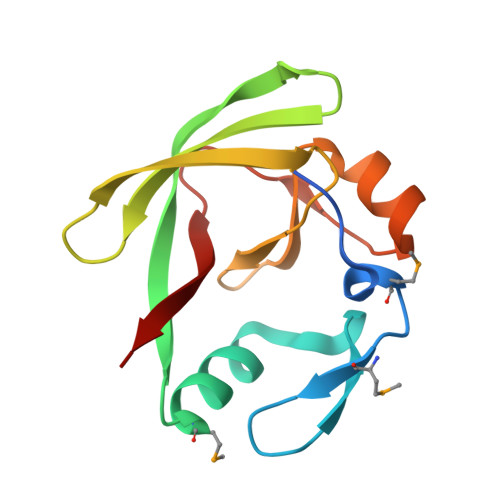
Structural basis of protein complex formation and reconfiguration by polyglutamine disease protein Ataxin-1 and Capicua
Kim, E., Lu, H.-C., Zoghbi, H.Y., Song, J.-J.(2013) Genes Dev 27: 590-595
- PubMed: 23512657
- DOI: https://doi.org/10.1101/gad.212068.112
- Primary Citation of Related Structures:
4J2J, 4J2L - PubMed Abstract:
Spinocerebellar ataxia type 1 (SCA1) is a dominantly inherited neurodegenerative disease caused by polyglutamine expansion in Ataxin-1 (ATXN1). ATXN1 binds to the transcriptional repressor Capicua (CIC), and the interaction plays a critical role in SCA1 pathogenesis whereby reducing CIC levels rescues SCA1-like phenotypes in a mouse model. The ATXN1/HBP1 (AXH) domain of ATXN1 mediates its homodimerization as well as the interaction with CIC. Here, we present the crystal structure of ATXN1's AXH domain bound to CIC and show that the binding pocket of the AXH domain to CIC overlaps with the homodimerization pocket of the AXH domain. Thus, the binding to CIC disrupts the homodimerization of ATXN1. Furthermore, the binding of CIC reconfigures the complex to allow another form of dimerization mediated by CIC, showing the intricacy of protein complex formation and reconfiguration by ATXN1 and CIC. Identifying the surfaces mediating the interactions between CIC and ATXN1 reveals a critical role for CIC in the reconfiguration of the AXH dimers and might provide insight into ways to target the ATXN1/CIC interactions to modulate SCA1 pathogenesis.
- Department of Biological Sciences, Graduate School of Nanoscience and Technology (World Class University), KAIST (Korea Advanced Institute of Science and Technology) Institute for the BioCentury, KAIST, Daejeon 305-701, Korea.
Organizational Affiliation: