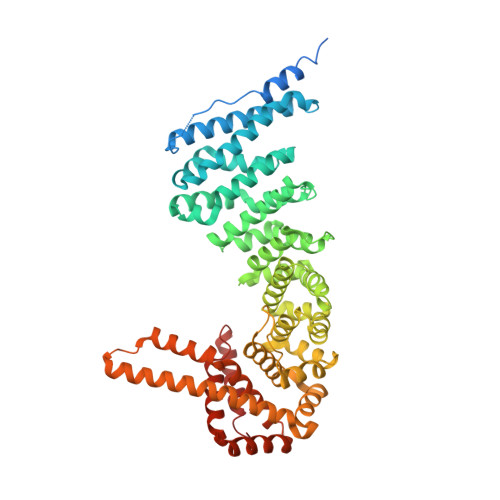
The structure of full-length human CTNNBL1 reveals a distinct member of the armadillo-repeat protein family.
Huang, X., Wang, G., Wu, Y., Du, Z.(2013) Acta Crystallogr D Biol Crystallogr 69: 1598-1608
- PubMed: 23897482
- DOI: https://doi.org/10.1107/S0907444913011360
- Primary Citation of Related Structures:
4HM9, 4HNM - PubMed Abstract:
Catenin-β-like protein 1 (CTNNBL1) is a highly conserved protein with multiple functions, one of which is to act as an interaction partner of the antibody-diversification enzyme activation-induced cytidine deaminase (AID) for its nuclear import and subnuclear trafficking. Here, the crystal structure of full-length human CTNNBL1 is reported. The protein contains six armadillo (ARM) repeats that pack into a superhelical ARM domain. This ARM domain is unique within the ARM protein family owing to the presence of several unusual structural features. Moreover, CTNNBL1 contains significant and novel non-ARM structures flanking both ends of the central ARM domain. A strong continuous hydrophobic core runs through the whole structure, indicating that the ARM and non-ARM structures fold together to form an integral structure. This structure defines a highly restrictive and discriminatory protein-binding groove that is not observed in other ARM proteins. The presence of a cluster of histidine residues in the groove implies a pH-sensitive histidine-mediated mechanism that may regulate protein binding activity. The many unique structural features of CTNNBL1 establish it as a distinct member of the ARM protein family. The structure provides critical insights into the molecular interactions between CTNNBL1 and its protein partners, especially AID.
- Department of Computer Science, Southern Illinois University, 1000 Faner Drive, Carbondale, IL 62901, USA.
Organizational Affiliation: