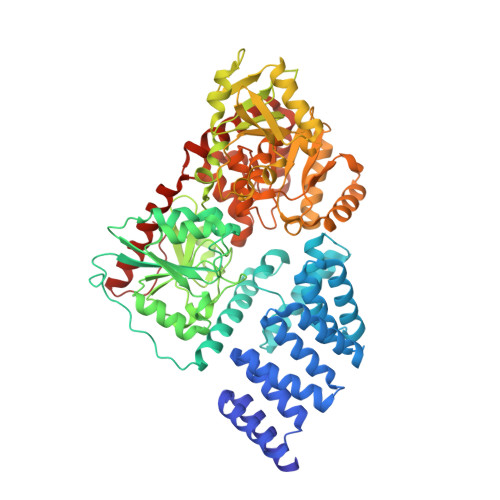
Structural snapshots of the reaction coordinate for O-GlcNAc transferase.
Lazarus, M.B., Jiang, J., Gloster, T.M., Zandberg, W.F., Whitworth, G.E., Vocadlo, D.J., Walker, S.(2012) Nat Chem Biol 8: 966-968
- PubMed: 23103939
- DOI: https://doi.org/10.1038/nchembio.1109
- Primary Citation of Related Structures:
4GYW, 4GYY, 4GZ3, 4GZ5, 4GZ6 - PubMed Abstract:
Visualization of the reaction coordinate undertaken by glycosyltransferases has remained elusive but is critical for understanding this important class of enzyme. Using substrates and substrate mimics, we describe structural snapshots of all species along the kinetic pathway for human O-linked β-N-acetylglucosamine transferase (O-GlcNAc transferase), an intracellular enzyme that catalyzes installation of a dynamic post-translational modification. The structures reveal key features of the mechanism and show that substrate participation is important during catalysis.
- Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts, USA.
Organizational Affiliation: