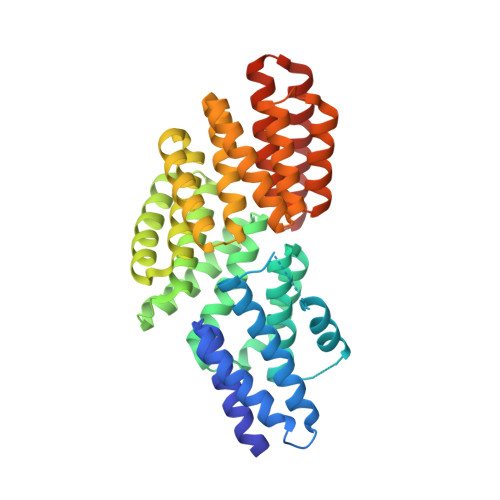
Conformational change-induced repeat domain expansion regulates rap phosphatase quorum-sensing signal receptors.
Parashar, V., Jeffrey, P.D., Neiditch, M.B.(2013) PLoS Biol 11: e1001512-e1001512
- PubMed: 23526881
- DOI: https://doi.org/10.1371/journal.pbio.1001512
- Primary Citation of Related Structures:
4GYO, 4I1A - PubMed Abstract:
The large family of Gram-positive quorum-sensing receptors known as the RNPP proteins consists of receptors homologous to the Rap, NprR, PlcR, and PrgX proteins that are regulated by imported oligopeptide autoinducers. Rap proteins are phosphatases and transcriptional anti-activators, and NprR, PlcR, and PrgX proteins are DNA binding transcription factors. Despite their obvious importance, the mechanistic basis of oligopeptide receptor regulation is largely unknown. Here, we report the X-ray crystal structure of the Bacillus subtilis quorum-sensing receptor RapJ in complex with the centrally important oligopeptide autoinducer competence and sporulation factor (CSF, also termed PhrC), a member of the Phr family of quorum-sensing signals. Furthermore, we present the crystal structure of RapI. Comparison of the RapJ-PhrC, RapI, RapH-Spo0F, and RapF-ComA(C) crystal structures reveals the mechanistic basis of Phr activity. More specifically, when complexed with target proteins, Rap proteins consist of a C-terminal tetratricopeptide repeat (TPR) domain connected by a flexible helix-containing linker to an N-terminal 3-helix bundle. In the absence of a target protein or regulatory peptide, the Rap protein 3-helix bundle adopts different conformations. However, in the peptide-bound conformation, the Rap protein N-terminal 3-helix bundle and linker undergo a radical conformational change, form TPR-like folds, and merge with the existing C-terminal TPR domain. To our knowledge, this is the first example of conformational change-induced repeat domain expansion. Furthermore, upon Phr binding, the entire Rap protein is compressed along the TPR superhelical axis, generating new intramolecular contacts that lock the Rap protein in an inactive state. The fact that Rap proteins are conformationally flexible is surprising considering that it is accepted dogma that TPR proteins do not undergo large conformational changes. Repeat proteins are widely used as scaffolds for the development of designed affinity reagents, and we propose that Rap proteins could be used as scaffolds for engineering novel ligand-switchable affinity reagents.
- Department of Microbiology and Molecular Genetics, UMDNJ-New Jersey Medical School, Newark, New Jersey, United States of America.
Organizational Affiliation: