The crystal structure of acyl carrier protein phosphodiesterase from Yersinia pestis CO92 in complex with FMN.
Tan, K., Gu, M., Kwon, K., Anderson, W.F., Joachimiak, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| FMN-dependent NADH-azoreductase | 204 | Yersinia pestis CO92 | Mutation(s): 0 Gene Names: azoR, y2010, YPO2323, YP_2110 EC: 1.7 (PDB Primary Data), 1.6.5 (UniProt), 1.7.1.17 (UniProt) | 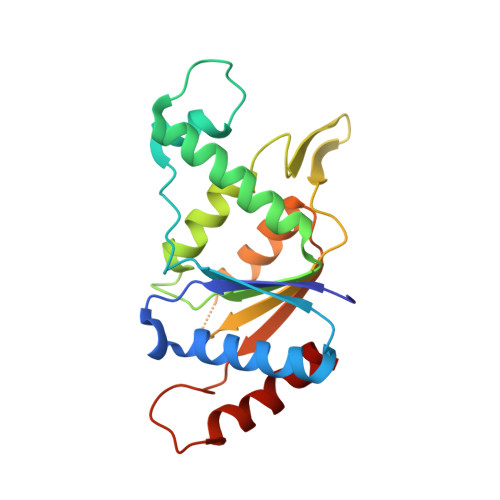 | |
UniProt | |||||
Find proteins for Q8ZE60 (Yersinia pestis) Explore Q8ZE60 Go to UniProtKB: Q8ZE60 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8ZE60 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 12P Query on 12P | E [auth A] | DODECAETHYLENE GLYCOL C24 H50 O13 WRZXKWFJEFFURH-UHFFFAOYSA-N |  | ||
| FMN Query on FMN | B [auth A], C [auth A], D [auth A] | FLAVIN MONONUCLEOTIDE C17 H21 N4 O9 P FVTCRASFADXXNN-SCRDCRAPSA-N |  | ||
| GOL Query on GOL | F [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| IMD Query on IMD | G [auth A] | IMIDAZOLE C3 H5 N2 RAXXELZNTBOGNW-UHFFFAOYSA-O |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 51.825 | α = 90 |
| b = 120.388 | β = 90 |
| c = 60.074 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| MOLREP | phasing |
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |