Fluoride Inhibition of Sporosarcina Pasteurii Urease: Structure and Thermodynamics.
Benini, S., Cianci, M., Mazzei, L., Ciurli, S.(2014) J Biol Inorg Chem 19: 1243
- PubMed: 25113581
- DOI: https://doi.org/10.1007/s00775-014-1182-x
- Primary Citation of Related Structures:
4CEU, 4CEX - PubMed Abstract:
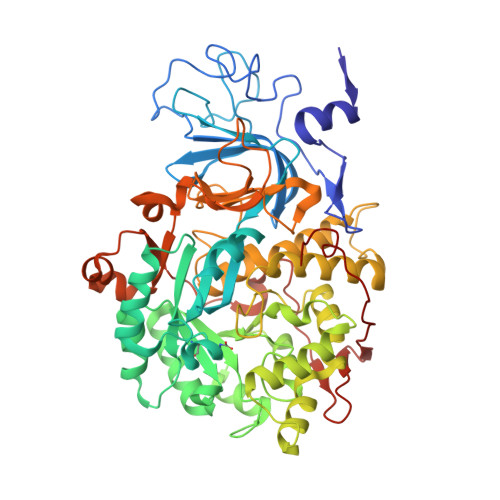
Urease is a nickel-dependent enzyme and a virulence factor for ureolytic bacterial human pathogens, but it is also necessary to convert urea, the most worldwide used fertilizer, into forms of nitrogen that can be taken up by crop plants. A strategy to control the activity of urease for medical and agricultural applications is to use enzyme inhibitors. Fluoride is a known urease inhibitor, but the structural basis of its mode of inhibition is still undetermined. Here, kinetic studies on the fluoride-induced inhibition of urease from Sporosarcina pasteurii, a widespread and highly ureolytic soil bacterium, were performed using isothermal titration calorimetry and revealed a mixed competitive and uncompetitive mechanism. The pH dependence of the inhibition constants, investigated in the 6.5-8.0 range, reveals a predominant uncompetitive mechanism that increases by increasing the pH, and a lesser competitive inhibition that increases by lowering the pH. Ten crystal structures of the enzyme were independently determined using five crystals of the native form and five crystals of the protein crystallized in the presence of fluoride. The analysis of these structures revealed the presence of two fluoride anions coordinated to the Ni(II) ions in the active site, in terminal and bridging positions. The present study consistently supports an interaction of fluoride with the nickel centers in the urease active site in which one fluoride competitively binds to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while another fluoride uncompetitively substitutes the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea.
- Faculty of Science and Technology, Free University of Bolzano, Piazza Università 5, 39100, Bolzano, Italy, stefano.benini@unibz.it.
Organizational Affiliation: