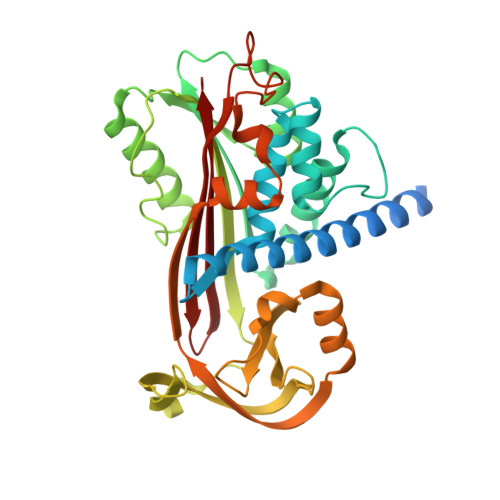
Structural Basis for Catalytic Activation of Protein Z-Dependent Protease Inhibitor (Zpi) by Protein Z.
Huang, X., Yan, Y., Tu, Y., Gatti, J., Broze, G.J.J., Zhou, A., Olson, S.T.(2012) Blood 120: 1726
- PubMed: 22786881
- DOI: https://doi.org/10.1182/blood-2012-03-419598
- Primary Citation of Related Structures:
4AFX, 4AJT, 4AJU - PubMed Abstract:
The anticoagulant serpin, protein Z-dependent protease inhibitor (ZPI), is catalytically activated by its cofactor, protein Z (PZ), to regulate the function of blood coagulation factor Xa on membrane surfaces. The X-ray structure of the ZPI-PZ complex has shown that PZ binds to a unique site on ZPI centered on helix G. In the present study, we show by Ala-scanning mutagenesis of the ZPI-binding interface, together with native PAGE and kinetic analyses of PZ binding to ZPI, that Tyr240 and Asp293 of ZPI are crucial hot spots for PZ binding. Complementary studies with protein Z-protein C chimeras show the importance of both pseudocatalytic and EGF2 domains of PZ for the critical ZPI interactions. To understand how PZ acts catalytically, we analyzed the interaction of reactive loop-cleaved ZPI (cZPI) with PZ and determined the cZPI X-ray structure. The cZPI structure revealed changes in helices A and G of the PZ-binding site relative to native ZPI that rationalized an observed 6-fold loss in PZ affinity and PZ catalytic action. These findings identify the key determinants of catalytic activation of ZPI by PZ and suggest novel strategies for ameliorating hemophilic states through drugs that disrupt the ZPI-PZ interaction.
- Center for Molecular Biology of Oral Diseases, University of Illinois at Chicago, Chicago, IL 60612, USA.
Organizational Affiliation: