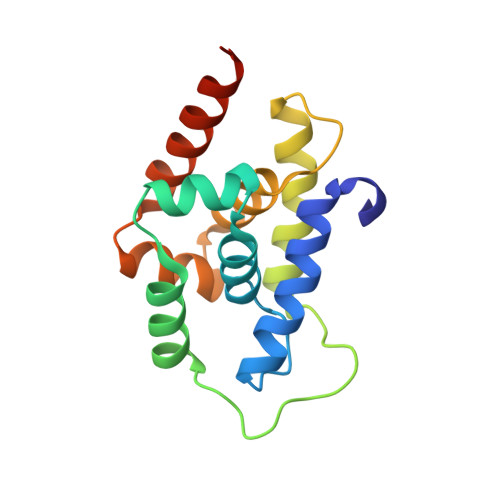
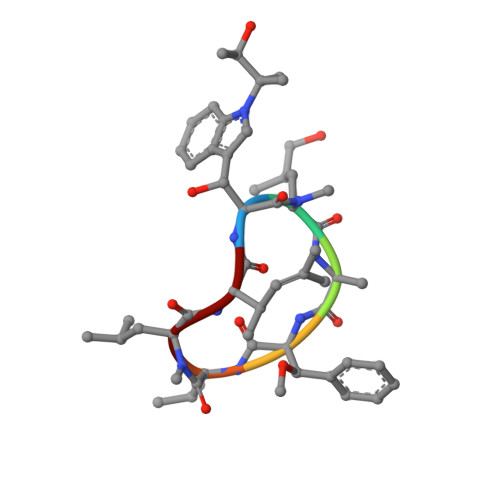
Structural basis of mycobacterial inhibition by cyclomarin A
Vasudevan, D., Rao, S.P.S., Noble, C.G.(2013) J Biological Chem 288: 30883-30891
- PubMed: 24022489
- DOI: https://doi.org/10.1074/jbc.M113.493767
- Primary Citation of Related Structures:
3WDB, 3WDC, 3WDD, 3WDE - PubMed Abstract:
Cyclomarin A (CymA) was identified as a mycobactericidal compound targeting ClpC1. However, the target was identified based on pulldown experiments and in vitro binding data, without direct functional evidence in mycobacteria. Here we show that CymA specifically binds to the N-terminal domain of ClpC1. In addition we have determined the co-crystal structure of CymA bound to the N-terminal domain of ClpC1 to high resolution. Based on the structure of the complex several mutations were engineered into ClpC1, which showed reduced CymA binding in vitro. The ClpC1 mutants were overexpressed in mycobacteria and two showed resistance to CymA, providing the first direct evidence that ClpC1 is the target of CymA. Phe(80) is important in vitro and in cells for the ClpC1-CymA interaction and this explains why other bacteria are resistant to CymA. A model for how CymA binding to the N-terminal domain of ClpC1 leads to uncontrolled proteolysis by the associated ClpP protease machinery is discussed.
- From the Novartis Institute for Tropical Diseases, 05-01 Chromos, Singapore 138670.
Organizational Affiliation: