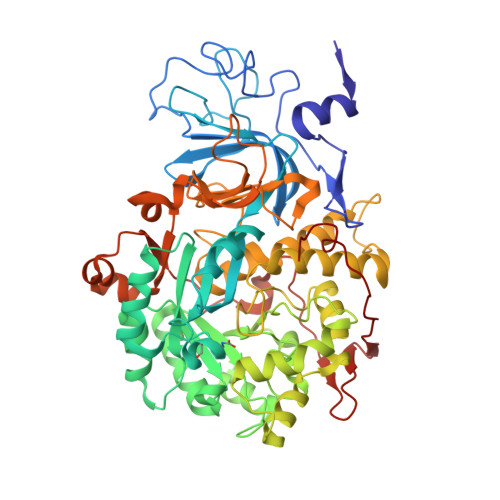
A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels.
Benini, S., Rypniewski, W.R., Wilson, K.S., Miletti, S., Ciurli, S., Mangani, S.(1999) Structure 7: 205-216
- PubMed: 10368287
- DOI: https://doi.org/10.1016/S0969-2126(99)80026-4
- Primary Citation of Related Structures:
2UBP, 3UBP - PubMed Abstract:
Urease catalyzes the hydrolysis of urea, the final step of organic nitrogen mineralization, using a bimetallic nickel centre. The role of the active site metal ions and amino acid residues has not been elucidated to date. Many pathologies are associated with the activity of ureolytic bacteria, and the efficiency of soil nitrogen fertilization with urea is severely decreased by urease activity. Therefore, the development of urease inhibitors would lead to a reduction of environmental pollution, to enhanced efficiency of nitrogen uptake by plants, and to improved therapeutic strategies for treatment of infections due to ureolytic bacteria. Structure-based design of urease inhibitors would require knowledge of the enzyme mechanism at the molecular level. The structures of native and inhibited urease from Bacillus pasteurii have been determined at a resolution of 2.0 A by synchrotron X-ray cryogenic crystallography. In the native enzyme, the coordination sphere of each of the two nickel ions is completed by a water molecule and a bridging hydroxide. A fourth water molecule completes a tetrahedral cluster of solvent molecules. The enzyme crystallized in the presence of phenylphosphorodiamidate contains the tetrahedral transition-state analogue diamidophosphoric acid, bound to the two nickel ions in an unprecedented mode. Comparison of the native and inhibited structures reveals two distinct conformations of the flap lining the active-site cavity. The mode of binding of the inhibitor, and a comparison between the native and inhibited urease structures, indicate a novel mechanism for enzymatic urea hydrolysis which reconciles the available structural and biochemical data.
- European Molecular Biology Laboratory, DESY, Notkestrasse 85 D-22603, Hamburg, Germany.
Organizational Affiliation: