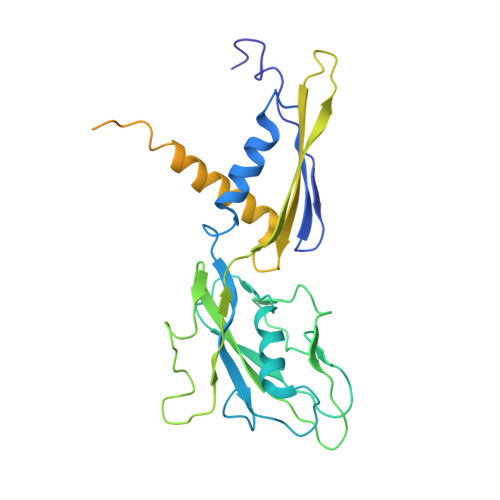
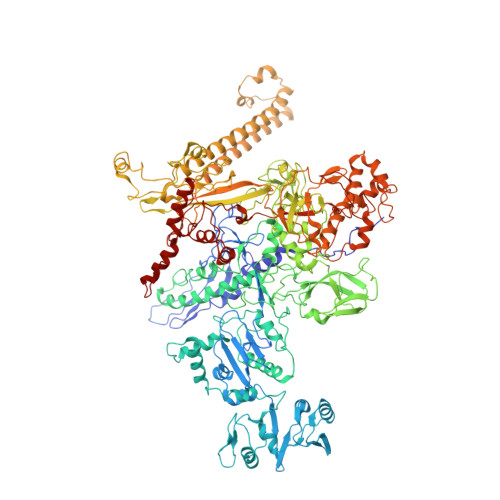
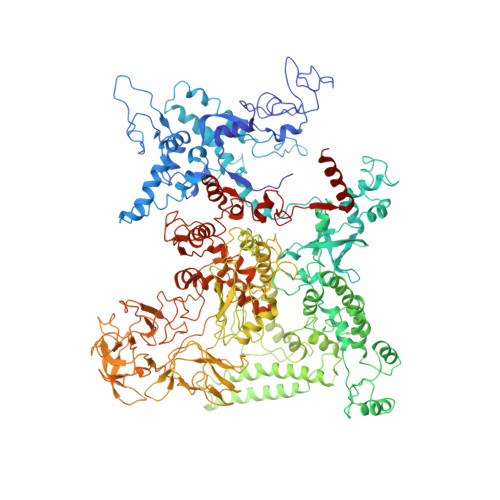
Complete structural model of Escherichia coli RNA polymerase from a hybrid approach.
Opalka, N., Brown, J., Lane, W.J., Twist, K.A., Landick, R., Asturias, F.J., Darst, S.A.(2010) PLoS Biol 8: e1000483
- PubMed: 20856905
- DOI: https://doi.org/10.1371/journal.pbio.1000483
- Primary Citation of Related Structures:
3LTI, 3LU0 - PubMed Abstract:
The Escherichia coli transcription system is the best characterized from a biochemical and genetic point of view and has served as a model system. Nevertheless, a molecular understanding of the details of E. coli transcription and its regulation, and therefore its full exploitation as a model system, has been hampered by the absence of high-resolution structural information on E. coli RNA polymerase (RNAP). We use a combination of approaches, including high-resolution X-ray crystallography, ab initio structural prediction, homology modeling, and single-particle cryo-electron microscopy, to generate complete atomic models of E. coli core RNAP and an E. coli RNAP ternary elongation complex. The detailed and comprehensive structural descriptions can be used to help interpret previous biochemical and genetic data in a new light and provide a structural framework for designing experiments to understand the function of the E. coli lineage-specific insertions and their role in the E. coli transcription program.
- The Rockefeller University, New York, New York, USA.
Organizational Affiliation: