Crystal structure of AraC family transcriptional regulator from Pseudomonas putida
Bagaria, A., Kumaran, D., Burley, S.K., Swaminathan, S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Transcriptional regulator, AraC family | 202 | Pseudomonas putida KT2440 | Mutation(s): 0 Gene Names: PP_3526, PP3526 | 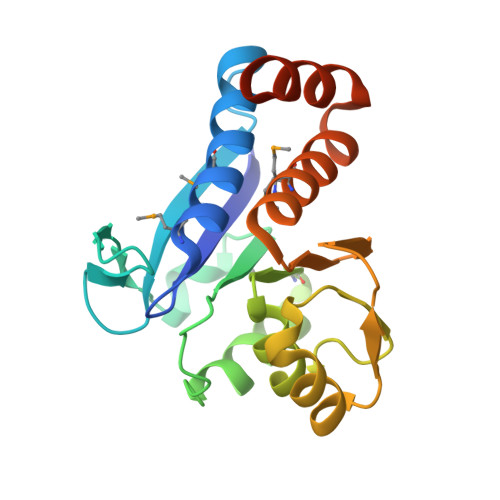 | |
UniProt | |||||
Find proteins for Q88H39 (Pseudomonas putida (strain ATCC 47054 / DSM 6125 / CFBP 8728 / NCIMB 11950 / KT2440)) Explore Q88H39 Go to UniProtKB: Q88H39 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q88H39 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | F [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| EDO Query on EDO | C [auth A], D [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| MG Query on MG | E [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 49.852 | α = 90 |
| b = 71.876 | β = 90 |
| c = 104.518 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CBASS | data collection |
| SHELXD | phasing |
| SHARP | phasing |
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |