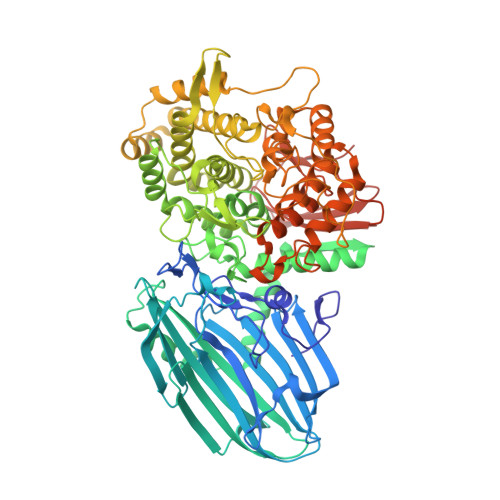
Structural and mutational analysis of substrate recognition in kojibiose phosphorylase
Okada, S., Yamamoto, T., Watanabe, H., Nishimoto, T., Chaen, H., Fukuda, S., Wakagi, T., Fushinobu, S.(2014) FEBS J 281: 778-786
- PubMed: 24255995
- DOI: https://doi.org/10.1111/febs.12622
- Primary Citation of Related Structures:
3WIQ, 3WIR - PubMed Abstract:
Glycoside hydrolase (GH) family 65 contains phosphorylases acting on maltose (Glc-α1,4-Glc), kojibiose (Glc-α1,2-Glc), trehalose (Glc-α1,α1,-Glc), and nigerose (Glc-α1,3-Glc). These phosphorylases can efficiently catalyze the reverse reactions with high specificities, and thus can be applied to the practical synthesis of α-glucosyl oligosaccharides. Here, we determined the crystal structures of kojibiose phosphorylase from Caldicellulosiruptor saccharolyticus in complex with glucose and phosphate and in complex with kojibiose and sulfate, providing the first structural insights into the substrate recognition of a glycoside hydrolase family 65 enzyme. The loop 3 region comprising the active site of kojibiose phosphorylase is significantly longer than the active sites of other enzymes, and three residues around this loop, Trp391, Glu392, and Thr417, recognize kojibiose. Various mutants mimicking the residue conservation patterns of other phosphorylases were constructed by mutation at these three residues. Activity measurements of the mutants against four substrates indicated that Trp391 and Glu392, especially the latter, are required for the kojibiose activity.
- Department of Biotechnology, University of Tokyo, Japan.
Organizational Affiliation: