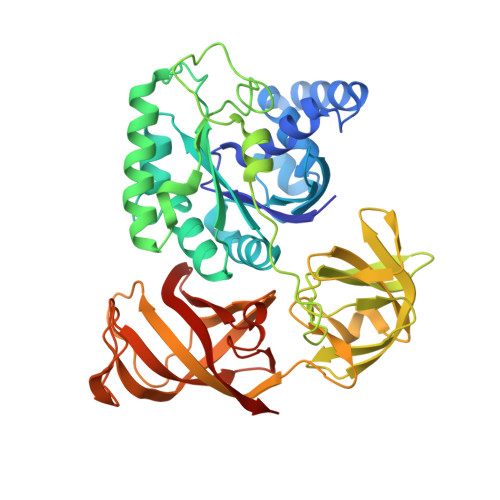
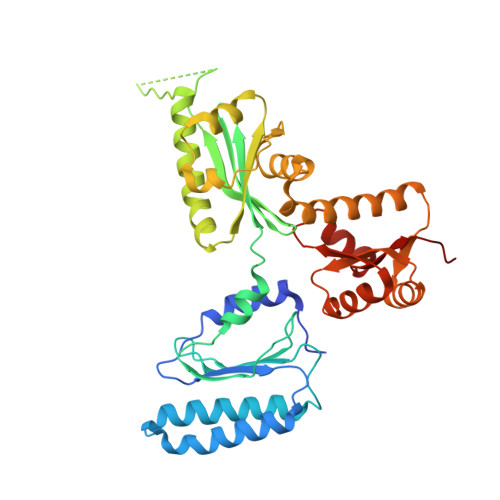
Structural basis for translation termination by archaeal RF1 and GTP-bound EF1alpha complex
Kobayashi, K., Saito, K., Ishitani, R., Ito, K., Nureki, O.(2012) Nucleic Acids Res 40: 9319-9328
- PubMed: 22772989
- DOI: https://doi.org/10.1093/nar/gks660
- Primary Citation of Related Structures:
3VMF - PubMed Abstract:
When a stop codon appears at the ribosomal A site, the class I and II release factors (RFs) terminate translation. In eukaryotes and archaea, the class I and II RFs form a heterodimeric complex, and complete the overall translation termination process in a GTP-dependent manner. However, the structural mechanism of the translation termination by the class I and II RF complex remains unresolved. In archaea, archaeal elongation factor 1 alpha (aEF1α), a carrier GTPase for tRNA, acts as a class II RF by forming a heterodimeric complex with archaeal RF1 (aRF1). We report the crystal structure of the aRF1·aEF1α complex, the first active class I and II RF complex. This structure remarkably resembles the tRNA·EF-Tu complex, suggesting that aRF1 is efficiently delivered to the ribosomal A site, by mimicking tRNA. It provides insights into the mechanism that couples GTP hydrolysis by the class II RF to stop codon recognition and peptidyl-tRNA hydrolysis by the class I RF. We discuss the different mechanisms by which aEF1α recognizes aRF1 and aPelota, another aRF1-related protein and molecular evolution of the three functions of aEF1α.
- Department of Biophysics and Biochemistry, Graduate School of Science, The University of Tokyo, 2-11-16 Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan.
Organizational Affiliation: