The Geometry of the Reactive Site and of the Peptide Groups in Trypsin, Trypsinogen and its Complexes with Inhibitors
Marquart, M., Walter, J., Deisenhofer, J., Bode, W., Huber, R.(1983) Acta Crystallogr B 39: 480
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(1983) Acta Crystallogr B 39: 480
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| TRYPSINOGEN | A [auth Z] | 229 | Bos taurus | Mutation(s): 0 EC: 3.4.21.4 | 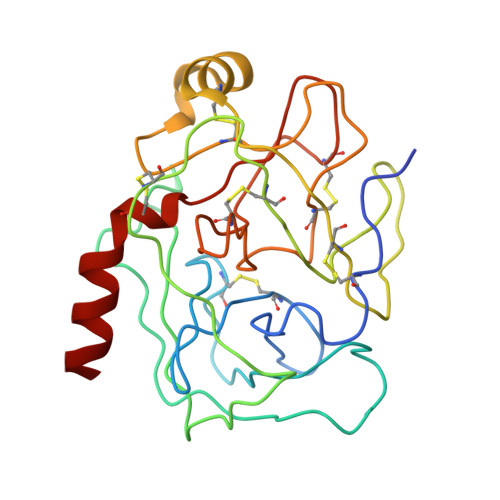 |
UniProt | |||||
Find proteins for P00760 (Bos taurus) Explore P00760 Go to UniProtKB: P00760 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00760 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| BOVINE PANCREATIC TRYPSIN INHIBITOR | B [auth I] | 58 | Bos taurus | Mutation(s): 0 | 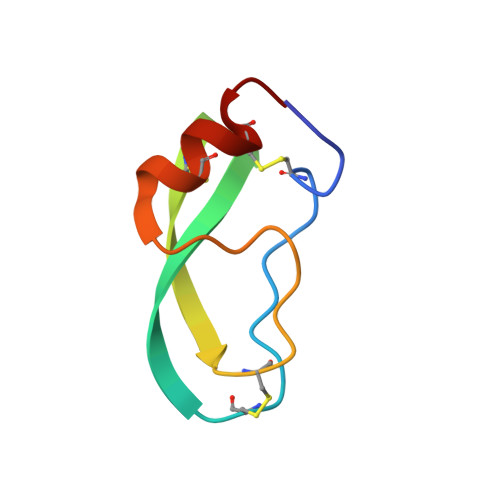 |
UniProt | |||||
Find proteins for P00974 (Bos taurus) Explore P00974 Go to UniProtKB: P00974 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00974 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ILE Query on ILE | C [auth Z] | ISOLEUCINE C6 H13 N O2 AGPKZVBTJJNPAG-WHFBIAKZSA-N |  | ||
| VAL Query on VAL | D [auth Z] | VALINE C5 H11 N O2 KZSNJWFQEVHDMF-BYPYZUCNSA-N |  | ||
| SO4 Query on SO4 | F [auth I], G [auth I] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| CA Query on CA | E [auth Z] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 75.5 | α = 90 |
| b = 84.4 | β = 90 |
| c = 122.9 | γ = 90 |