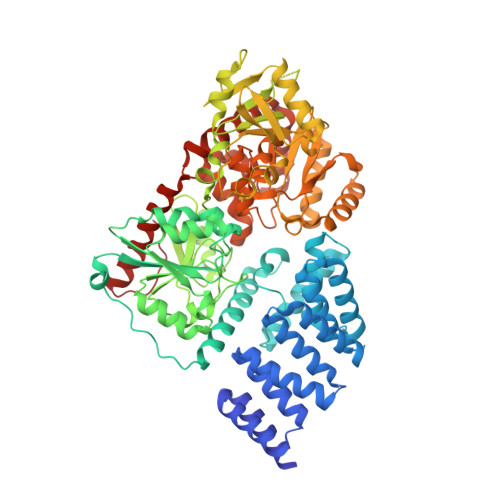
A neutral diphosphate mimic crosslinks the active site of human O-GlcNAc transferase.
Jiang, J., Lazarus, M.B., Pasquina, L., Sliz, P., Walker, S.(2011) Nat Chem Biol 8: 72-77
- PubMed: 22082911
- DOI: https://doi.org/10.1038/nchembio.711
- Primary Citation of Related Structures:
3TAX - PubMed Abstract:
Glycosyltransferases (Gtfs) catalyze the formation of a diverse array of glycoconjugates. Small-molecule inhibitors to manipulate Gtf activity in cells have long been sought as tools for understanding Gtf function. Success has been limited because of challenges in designing inhibitors that mimic the negatively charged diphosphate substrates. Here we report the mechanism of action of a small molecule that inhibits O-linked N-acetylglucosamine transferase (OGT), an essential human enzyme that modulates cell signaling pathways by catalyzing a unique intracellular post-translational modification, β-O-GlcNAcylation. The molecule contains a five-heteroatom dicarbamate core that functions as a neutral diphosphate mimic. One dicarbamate carbonyl reacts with an essential active site lysine that anchors the diphosphate of the nucleotide-sugar substrate. A nearby cysteine then reacts with the lysine adduct to form a carbonyl crosslink in the OGT active site. Though this unprecedented double-displacement mechanism reflects the unique architecture of the OGT active site, related dicarbamate scaffolds may inhibit other enzymes that bind nucleotide-containing substrates.
- Department of Microbiology and Immunobiology, Harvard Medical School, Boston, Massachusetts, USA.
Organizational Affiliation: