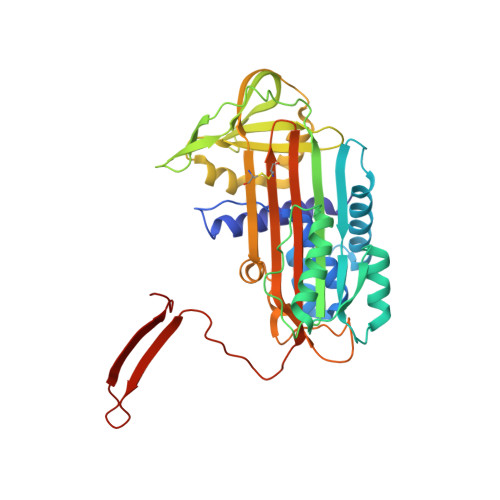
Molecular basis of alpha 1-antitrypsin deficiency revealed by the structure of a domain-swapped trimer.
Yamasaki, M., Sendall, T.J., Pearce, M.C., Whisstock, J.C., Huntington, J.A.(2011) EMBO Rep 12: 1011-1017
- PubMed: 21909074
- DOI: https://doi.org/10.1038/embor.2011.171
- Primary Citation of Related Structures:
3T1P - PubMed Abstract:
α(1)-Antitrypsin (α1AT) deficiency is a disease with multiple manifestations, including cirrhosis and emphysema, caused by the accumulation of stable polymers of mutant protein in the endoplasmic reticulum of hepatocytes. However, the molecular basis of misfolding and polymerization remain unknown. We produced and crystallized a trimeric form of α1AT that is recognized by an antibody specific for the pathological polymer. Unexpectedly, this structure reveals a polymeric linkage mediated by domain swapping the carboxy-terminal 34 residues. Disulphide-trapping and antibody-binding studies further demonstrate that runaway C-terminal domain swapping, rather than the s4A/s5A domain swap previously proposed, underlies polymerization of the common Z-mutant of α1AT in vivo.
- Department of Haematology, Cambridge Institute for Medical Research, University of Cambridge, Wellcome Trust/MRC Building, Hills Road, Cambridge CB2 0XY, UK.
Organizational Affiliation: