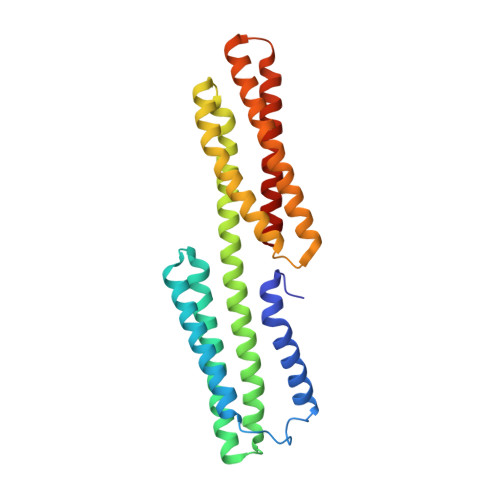
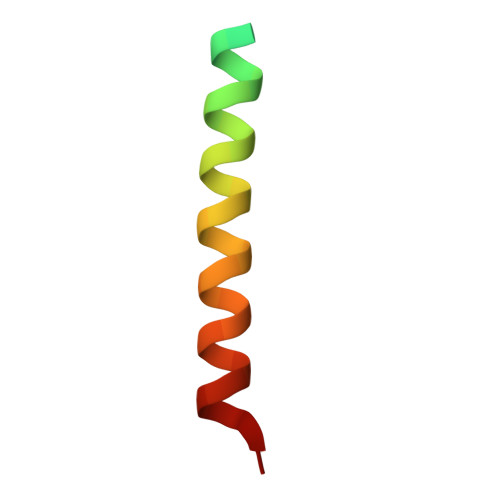
Intermolecular versus intramolecular interactions of the vinculin binding site 33 of talin.
Yogesha, S.D., Sharff, A., Bricogne, G., Izard, T.(2011) Protein Sci 20: 1471-1476
- PubMed: 21648001
- DOI: https://doi.org/10.1002/pro.671
- Primary Citation of Related Structures:
3S90 - PubMed Abstract:
The cytoskeletal proteins talin and vinculin are localized at cell-matrix junctions and are key regulators of cell signaling, adhesion, and migration. Talin couples integrins via its FERM domain to F-actin and is an important regulator of integrin activation and clustering. The 220 kDa talin rod domain comprises several four- and five-helix bundles that harbor amphipathic α-helical vinculin binding sites (VBSs). In its inactive state, the hydrophobic VBS residues involved in binding to vinculin are buried within these helix bundles, and the mechanical force emanating from bound integrin receptors is thought necessary for their release and binding to vinculin. The crystal structure of a four-helix bundle of talin that harbors one of these VBSs, coined VBS33, was recently determined. Here we report the crystal structure of VBS33 in complex with vinculin at 2 Å resolution. Notably, comparison of the apo and vinculin bound structures shows that intermolecular interactions of the VBS33 α-helix with vinculin are more extensive than the intramolecular interactions of the VBS33 within the talin four-helix bundle.
- Cell Adhesion Laboratory, Department of Cancer Biology, The Scripps Research Institute, Jupiter, FL 33458, USA.
Organizational Affiliation: