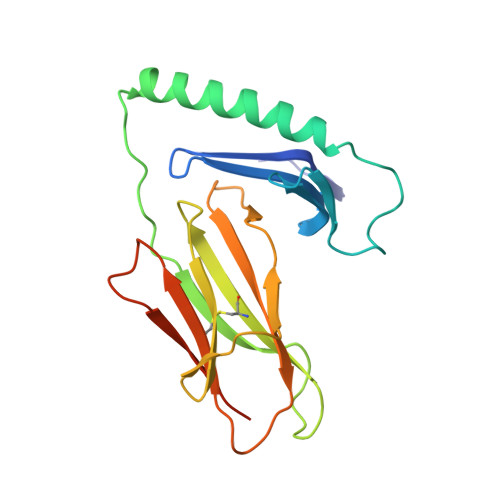
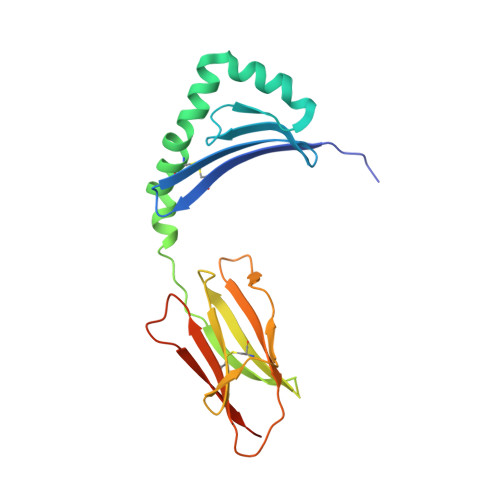
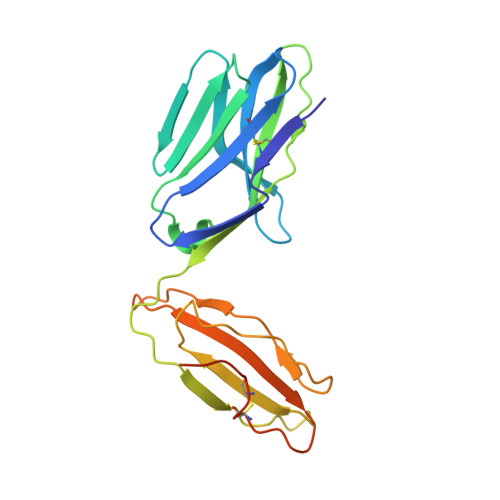
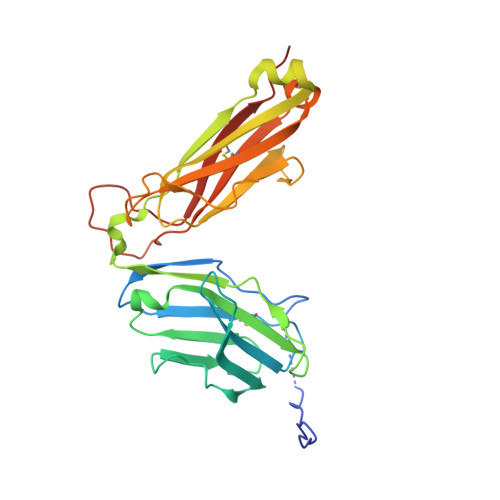
A highly tilted binding mode by a self-reactive T cell receptor results in altered engagement of peptide and MHC.
Sethi, D.K., Schubert, D.A., Anders, A.K., Heroux, A., Bonsor, D.A., Thomas, C.P., Sundberg, E.J., Pyrdol, J., Wucherpfennig, K.W.(2011) J Exp Medicine 208: 91-102
- PubMed: 21199956
- DOI: https://doi.org/10.1084/jem.20100725
- Primary Citation of Related Structures:
3PL6 - PubMed Abstract:
Self-reactive T cells that escape elimination in the thymus can cause autoimmune pathology, and it is therefore important to understand the structural mechanisms of self-antigen recognition. We report the crystal structure of a T cell receptor (TCR) from a patient with relapsing-remitting multiple sclerosis that engages its self-peptide-major histocompatibility complex (pMHC) ligand in an unusual manner. The TCR is bound in a highly tilted orientation that prevents interaction of the TCR-α chain with the MHC class II β chain helix. In this structure, only a single germline-encoded TCR loop engages the MHC protein, whereas in most other TCR-pMHC structures all four germline-encoded TCR loops bind to the MHC helices. The tilted binding mode also prevents peptide contacts by the short complementarity-determining region (CDR) 3β loop, and interactions that contribute to peptide side chain specificity are focused on the CDR3α loop. This structure is the first example in which only a single germline-encoded TCR loop contacts the MHC helices. Furthermore, the reduced interaction surface with the peptide may facilitate TCR cross-reactivity. The structural alterations in the trimolecular complex are distinct from previously characterized self-reactive TCRs, indicating that there are multiple unusual ways for self-reactive TCRs to bind their pMHC ligand.
- Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Boston, MA 02115, USA.
Organizational Affiliation: