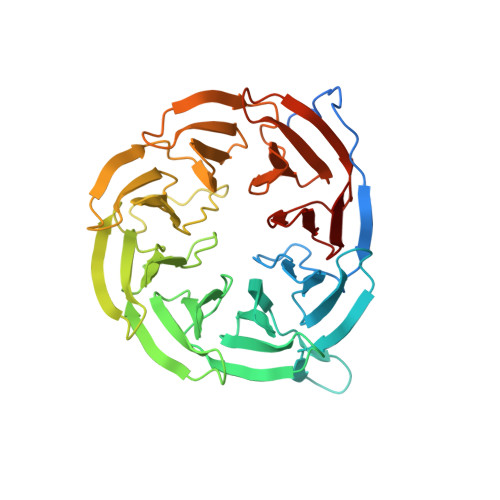
Crystal structure of Escherichia coli BamB, a lipoprotein component of the beta-barrel assembly machinery complex, native crystals
Kim, K.H., Paetzel, M.(2011) J Mol Biology 406: 667-678
- PubMed: 21168416
- DOI: https://doi.org/10.1016/j.jmb.2010.12.020
- Primary Citation of Related Structures:
3P1L - PubMed Abstract:
In Gram-negative bacteria, the BAM (β-barrel assembly machinery) complex catalyzes the essential process of assembling outer membrane proteins. The BAM complex in Escherichia coli consists of five proteins: one β-barrel membrane protein, BamA, and four lipoproteins, BamB, BamC, BamD, and BamE. Despite their role in outer membrane protein biogenesis, there is currently a lack of functional and structural information on the lipoprotein components of the BAM complex. Here, we report the first crystal structure of BamB, the largest and most functionally characterized lipoprotein component of the BAM complex. The crystal structure shows that BamB has an eight-bladed β-propeller structure, with four β-strands making up each blade. Mapping onto the structure the residues previously shown to be important for BamA interaction reveals that these residues, despite being far apart in the amino acid sequence, are localized to form a continuous solvent-exposed surface on one side of the β-propeller. Found on the same side of the β-propeller is a cluster of residues conserved among BamB homologs. Interestingly, our structural comparison study suggests that other proteins with a BamB-like fold often participate in protein or ligand binding, and that the binding interface on these proteins is located on the surface that is topologically equivalent to where the conserved residues and the residues that are important for BamA interaction are found on BamB. Our structural and bioinformatic analyses, together with previous biochemical data, provide clues to where the BamA and possibly a substrate interaction interface may be located on BamB.
- Department of Molecular Biology and Biochemistry, Simon Fraser University, South Science Building, 8888 University Drive, Burnaby, British Columbia, Canada.
Organizational Affiliation: