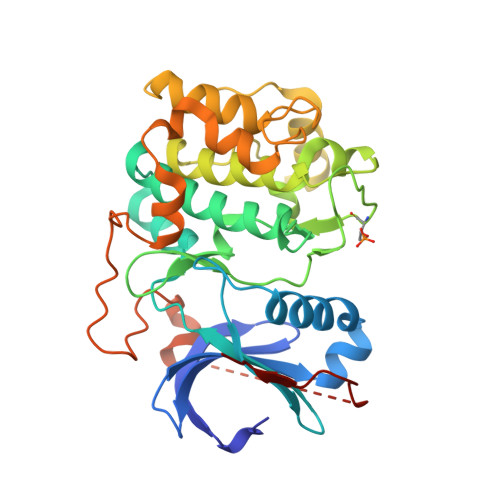
Discovery of pyrrolopyrimidine inhibitors of Akt.
Blake, J.F., Kallan, N.C., Xiao, D., Xu, R., Bencsik, J.R., Skelton, N.J., Spencer, K.L., Mitchell, I.S., Woessner, R.D., Gloor, S.L., Risom, T., Gross, S.D., Martinson, M., Morales, T.H., Vigers, G.P., Brandhuber, B.J.(2010) Bioorg Med Chem Lett 20: 5607-5612
- PubMed: 20810279
- DOI: https://doi.org/10.1016/j.bmcl.2010.08.053
- Primary Citation of Related Structures:
3OCB - PubMed Abstract:
The discovery and optimization of a series of pyrrolopyrimidine based protein kinase B (Pkb/Akt) inhibitors discovered via HTS and structure based drug design is reported. The compounds demonstrate potent inhibition of all three Akt isoforms and knockdown of phospho-PRAS40 levels in LNCaP cells and tumor xenografts.
- Array BioPharma Inc, 3200 Walnut Street, Boulder, CO 80301, USA. jblake@arraybiopharma.com
Organizational Affiliation: