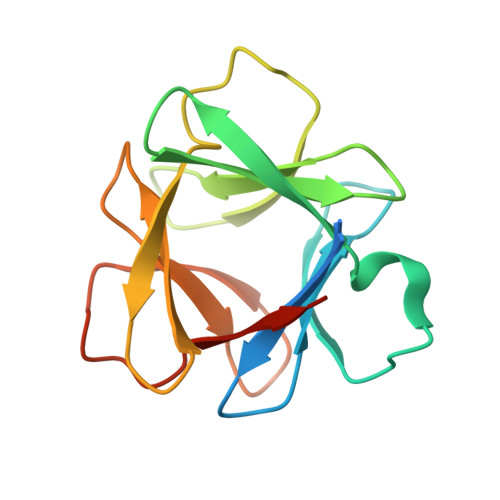
Experimental support for the evolution of symmetric protein architecture from a simple peptide motif.
Lee, J., Blaber, M.(2011) Proc Natl Acad Sci U S A 108: 126-130
- PubMed: 21173271
- DOI: https://doi.org/10.1073/pnas.1015032108
- Primary Citation of Related Structures:
3O49, 3O4A, 3O4B, 3O4C, 3O4D, 3OGF, 3OL0 - PubMed Abstract:
The majority of protein architectures exhibit elements of structural symmetry, and "gene duplication and fusion" is the evolutionary mechanism generally hypothesized to be responsible for their emergence from simple peptide motifs. Despite the central importance of the gene duplication and fusion hypothesis, experimental support for a plausible evolutionary pathway for a specific protein architecture has yet to be effectively demonstrated. To address this question, a unique "top-down symmetric deconstruction" strategy was utilized to successfully identify a simple peptide motif capable of recapitulating, via gene duplication and fusion processes, a symmetric protein architecture (the threefold symmetric β-trefoil fold). The folding properties of intermediary forms in this deconstruction agree precisely with a previously proposed "conserved architecture" model for symmetric protein evolution. Furthermore, a route through foldable sequence-space between the simple peptide motif and extant protein fold is demonstrated. These results provide compelling experimental support for a plausible evolutionary pathway of symmetric protein architecture via gene duplication and fusion processes.
- Department of Biomedical Sciences, Florida State University, Tallahassee FL 32306-4300, USA.
Organizational Affiliation: