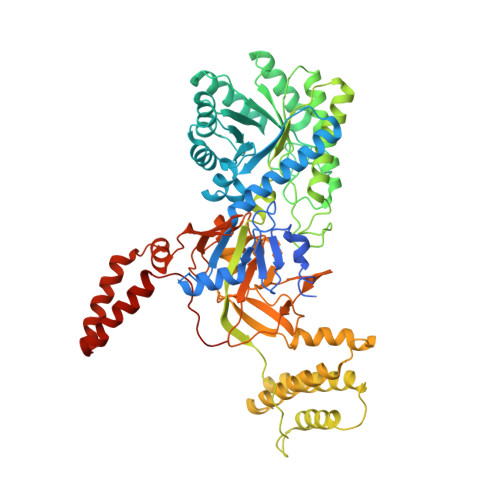
Structures of bacterial biosynthetic arginine decarboxylases.
Forouhar, F., Lew, S., Seetharaman, J., Xiao, R., Acton, T.B., Montelione, G.T., Tong, L.(2010) Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 1562-1566
- PubMed: 21139196
- DOI: https://doi.org/10.1107/S1744309110040649
- Primary Citation of Related Structures:
3NZP, 3NZQ - PubMed Abstract:
Biosynthetic arginine decarboxylase (ADC; also known as SpeA) plays an important role in the biosynthesis of polyamines from arginine in bacteria and plants. SpeA is a pyridoxal-5'-phosphate (PLP)-dependent enzyme and shares weak sequence homology with several other PLP-dependent decarboxylases. Here, the crystal structure of PLP-bound SpeA from Campylobacter jejuni is reported at 3.0 Å resolution and that of Escherichia coli SpeA in complex with a sulfate ion is reported at 3.1 Å resolution. The structure of the SpeA monomer contains two large domains, an N-terminal TIM-barrel domain followed by a β-sandwich domain, as well as two smaller helical domains. The TIM-barrel and β-sandwich domains share structural homology with several other PLP-dependent decarboxylases, even though the sequence conservation among these enzymes is less than 25%. A similar tetramer is observed for both C. jejuni and E. coli SpeA, composed of two dimers of tightly associated monomers. The active site of SpeA is located at the interface of this dimer and is formed by residues from the TIM-barrel domain of one monomer and a highly conserved loop in the β-sandwich domain of the other monomer. The PLP cofactor is recognized by hydrogen-bonding, π-stacking and van der Waals interactions.
- Northeast Structural Genomics Consortium, New York, NY 10027, USA.
Organizational Affiliation: