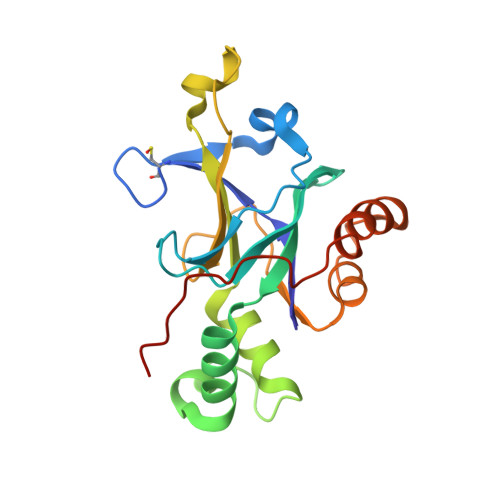
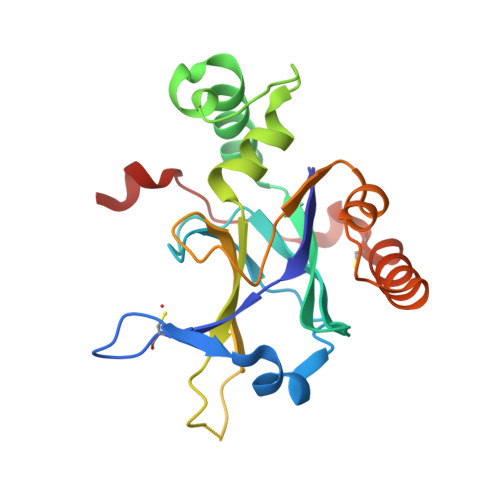
Enzyme inhibition by allosteric capture of an inactive conformation.
Lee, G.M., Shahian, T., Baharuddin, A., Gable, J.E., Craik, C.S.(2011) J Mol Biology 411: 999-1016
- PubMed: 21723875
- DOI: https://doi.org/10.1016/j.jmb.2011.06.032
- Primary Citation of Related Structures:
3NJQ - PubMed Abstract:
All members of the human herpesvirus protease (HHV Pr) family are active as weakly associating dimers but inactive as monomers. A small-molecule allosteric inhibitor of Kaposi's sarcoma-associated herpesvirus protease (KSHV Pr) traps the enzyme in an inactive monomeric state where the C-terminal helices are unfolded and the hydrophobic dimer interface is exposed. NMR titration studies demonstrate that the inhibitor binds to KSHV Pr monomers with low micromolar affinity. A 2.0-Å-resolution X-ray crystal structure of a C-terminal truncated KSHV Pr-inhibitor complex locates the binding pocket at the dimer interface and displays significant conformational perturbations at the active site, 15 Å from the allosteric site. NMR and CD data suggest that the small molecule inhibits human cytomegalovirus protease via a similar mechanism. As all HHV Prs are functionally and structurally homologous, the inhibitor represents a class of compounds that may be developed into broad-spectrum therapeutics that allosterically regulate enzymatic activity by disrupting protein-protein interactions.
- Department of Pharmaceutical Chemistry, University of California, San Francisco, CA 94158-2280, USA.
Organizational Affiliation: