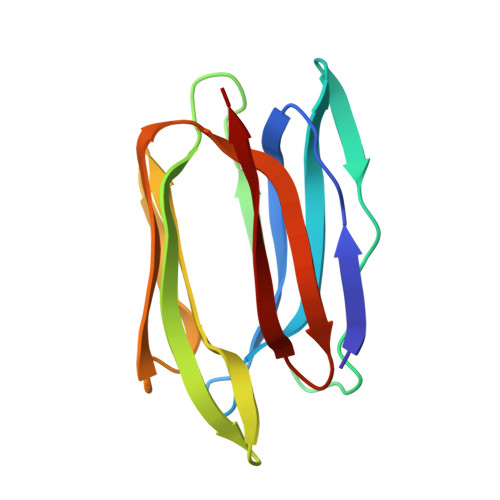
Characterization of the secondary binding sites of Maclura pomifera agglutinin by glycan array and crystallographic analyses.
Huang, J., Xu, Z., Wang, D., Ogata, C.M., Palczewski, K., Lee, X., Young, N.M.(2010) Glycobiology 20: 1643-1653
- PubMed: 20826825
- DOI: https://doi.org/10.1093/glycob/cwq118
- Primary Citation of Related Structures:
3LLY, 3LLZ, 3LM1 - PubMed Abstract:
The Maclura pomifera agglutinin (MPA) recognizes the T-antigen disaccharide Galβ1,3GalNAc mainly through interaction of the α-GalNAc moiety with its primary site, but the interactions of the two flanking subsites A and B with aglycones and substituents other than Gal, respectively, are not well understood. We therefore characterized the specificity of MPA in more detail by glycan microarray analysis and determined the crystal structures of MPA without ligand and in complexes with Galβ1,3GalNAc and p-nitrophenyl α-GalNAc. In both sugar complexes, pairs of ligands created inter-tetramer hydrogen-bond bridging networks. While subsite A showed increased affinity for hydrophobic aglycones, it also accommodated several sugar substituents. Notably, a GalNAc-O-tripeptide, a Tn-antigen mimic, showed lower affinity than these compounds in surface plasmon resonance (SPR) experiments. The glycan array data that showed subsite B accepted compounds in which the O3 position of the GalNAc was substituted with various sugars other than Gal, but substitutions at O6 led to inactivity. Additions to the Gal moiety of the disaccharide also had only small effects on reactivity. These results are all compatible with the features seen in the crystal structures.
- Department of Pharmacology, School of Medicine, Case Western Reserve University, 2109 Adelbert Rd, Cleveland, OH 44106, USA.
Organizational Affiliation: